Flexible Organic Photovoltaic-Powered Hydrogel Bioelectronic Dressing With Biomimetic Electrical Stimulation for Healing Infected Diabetic Wounds
- PMID: 38145346
- PMCID: PMC10933690
- DOI: 10.1002/advs.202307746
Flexible Organic Photovoltaic-Powered Hydrogel Bioelectronic Dressing With Biomimetic Electrical Stimulation for Healing Infected Diabetic Wounds
Abstract
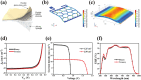
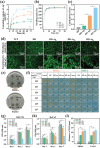
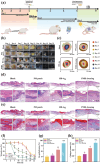
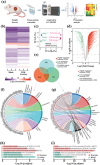
Electrical stimulation (ES) is proposed as a therapeutic solution for managing chronic wounds. However, its widespread clinical adoption is limited by the requirement of additional extracorporeal devices to power ES-based wound dressings. In this study, a novel sandwich-structured photovoltaic microcurrent hydrogel dressing (PMH dressing) is designed for treating diabetic wounds. This innovative dressing comprises flexible organic photovoltaic (OPV) cells, a flexible micro-electro-mechanical systems (MEMS) electrode, and a multifunctional hydrogel serving as an electrode-tissue interface. The PMH dressing is engineered to administer ES, mimicking the physiological injury current occurring naturally in wounds when exposed to light; thus, facilitating wound healing. In vitro experiments are performed to validate the PMH dressing's exceptional biocompatibility and robust antibacterial properties. In vivo experiments and proteomic analysis reveal that the proposed PMH dressing significantly accelerates the healing of infected diabetic wounds by enhancing extracellular matrix regeneration, eliminating bacteria, regulating inflammatory responses, and modulating vascular functions. Therefore, the PMH dressing is a potent, versatile, and effective solution for diabetic wound care, paving the way for advancements in wireless ES wound dressings.
Keywords: conducting hydrogel; electrical stimulation; flexible photovoltaic cells; proteomics; wound dressing.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A conductive multifunctional hydrogel dressing with the synergistic effect of ROS-scavenging and electroactivity for the treatment and sensing of chronic diabetic wounds.Acta Biomater. 2023 Sep 1;167:348-360. doi: 10.1016/j.actbio.2023.05.045. Epub 2023 Jun 2. Acta Biomater. 2023. PMID: 37270075
-
Two-Layered Biomimetic Flexible Self-Powered Electrical Stimulator for Promoting Wound Healing.Biomacromolecules. 2023 Mar 13;24(3):1483-1496. doi: 10.1021/acs.biomac.2c01520. Epub 2023 Feb 20. Biomacromolecules. 2023. PMID: 36802497
-
An all-in-one CO gas therapy-based hydrogel dressing with sustained insulin release, anti-oxidative stress, antibacterial, and anti-inflammatory capabilities for infected diabetic wounds.Acta Biomater. 2022 Jul 1;146:49-65. doi: 10.1016/j.actbio.2022.04.043. Epub 2022 Apr 30. Acta Biomater. 2022. PMID: 35500813
-
[Research advances on multifunctional hydrogel dressings for treatment of diabetic chronic wounds].Zhonghua Shao Shang Za Zhi. 2021 Nov 20;37(11):1090-1098. doi: 10.3760/cma.j.cn501120-20210715-00249. Zhonghua Shao Shang Za Zhi. 2021. PMID: 34794262 Free PMC article. Review. Chinese.
-
Functional Hydrogels as Wound Dressing to Enhance Wound Healing.ACS Nano. 2021 Aug 24;15(8):12687-12722. doi: 10.1021/acsnano.1c04206. Epub 2021 Aug 10. ACS Nano. 2021. PMID: 34374515 Review.
Cited by
-
Electret-Inspired Charge-Injected Hydrogel for Scar-Free Healing of Bacterially Infected Burns Through Bioelectrical Stimulation and Immune Modulation.Adv Sci (Weinh). 2025 Apr;12(13):e2411889. doi: 10.1002/advs.202411889. Epub 2025 Feb 14. Adv Sci (Weinh). 2025. PMID: 39951351 Free PMC article.
-
An Intelligent and Conductive Hydrogel with Multiresponsive and ROS Scavenging Properties for Infection Prevention and Anti-Inflammatory Treatment Assisted by Electrical Stimulation for Diabetic Wound.Adv Sci (Weinh). 2025 Jun;12(23):e2500696. doi: 10.1002/advs.202500696. Epub 2025 May 8. Adv Sci (Weinh). 2025. PMID: 40344517 Free PMC article.
-
Endogenous electric field-driven neuro-immuno-regulatory scaffold for effective diabetic wound healing.Bioact Mater. 2025 Jan 25;47:266-282. doi: 10.1016/j.bioactmat.2025.01.024. eCollection 2025 May. Bioact Mater. 2025. PMID: 39925709 Free PMC article.
-
Multimodal Synergistic Strategies for Diabetic Wound Healing Using Glucose Oxidase Nanocomposites: Therapeutic Mechanisms and Nanomaterial Design.Int J Nanomedicine. 2025 May 2;20:5727-5762. doi: 10.2147/IJN.S515057. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40337147 Free PMC article.
-
Optimizing Flexible Microelectrode Designs for Enhanced Efficacy in Electrical Stimulation Therapy.Micromachines (Basel). 2024 Aug 30;15(9):1104. doi: 10.3390/mi15091104. Micromachines (Basel). 2024. PMID: 39337764 Free PMC article.
References
-
- Lopez A. D., Mathers C. D., Ezzati M., Jamison D. T., Murray C. J., Lancet 2006, 367, 1747. - PubMed
-
- Mude L., Sanapalli B. K. R., A. N. V, Singh S. K., Karri V. V. S. R., Drug Dev. Res. 2021, 82, 503. - PubMed
-
- a) Bardill J. R., Laughter M. R., Stager M., Liechty K. W., Krebs M. D., Zgheib C., Acta Biomater. 2022, 138, 73; - PMC - PubMed
- b) Malone‐Povolny M. J., Maloney S. E., Schoenfisch M. H., Adv. Healthcare Mater. 2019, 8, e1801210; - PMC - PubMed
- c) Jiang J., Li X., Li H., Lv X., Xu Y., Hu Y., Song Y., Shao J., Li S., Yang D., J. Mater. Chem. B 2023, 11, 6746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
