Multiplexed Surface Electrode Arrays Based on Metal Oxide Thin-Film Electronics for High-Resolution Cortical Mapping
- PMID: 38145348
- PMCID: PMC10933637
- DOI: 10.1002/advs.202308507
Multiplexed Surface Electrode Arrays Based on Metal Oxide Thin-Film Electronics for High-Resolution Cortical Mapping
Abstract
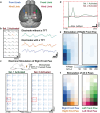
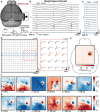
Electrode grids are used in neuroscience research and clinical practice to record electrical activity from the surface of the brain. However, existing passive electrocorticography (ECoG) technologies are unable to offer both high spatial resolution and wide cortical coverage, while ensuring a compact acquisition system. The electrode count and density are restricted by the fact that each electrode must be individually wired. This work presents an active micro-electrocorticography (µECoG) implant that tackles this limitation by incorporating metal oxide thin-film transistors (TFTs) into a flexible electrode array, allowing to address multiple electrodes through a single shared readout line. By combining the array with an incremental-ΔΣ readout integrated circuit (ROIC), the system is capable of recording from up to 256 electrodes virtually simultaneously, thanks to the implemented 16:1 time-division multiplexing scheme, offering lower noise levels than existing active µECoG arrays. In vivo validation is demonstrated acutely in mice by recording spontaneous activity and somatosensory evoked potentials over a cortical surface of ≈8×8 mm2 . The proposed neural interface overcomes the wiring bottleneck limiting ECoG arrays, holding promise as a powerful tool for improved mapping of the cerebral cortex and as an enabling technology for future brain-machine interfaces.
Keywords: a-IGZO; electrocorticography; electrode arrays; flexible electronics; thin-film transistors; time-division multiplexing; µECoGs.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Health Organization, Neurological Disorders: Public Health Challenges, 2006.
-
- DiLuca M., Olesen J., Neuron 2014, 82, 1205. - PubMed
-
- Engel A. K., Moll C. K. E., Fried I., Ojemann G. A., Nat. Rev. Neurosci. 2005, 6, 35. - PubMed
-
- Bronstein J. M., Tagliati M., Alterman R. L., Lozano A. M., Volkmann J., Stefani A., Horak F. B., Okun M. S., Foote K. D., Krack P., Pahwa R., Henderson J. M., Hariz M. I., Bakay R. A., Rezai A., Marks W. J., Moro E., Vitek J. L., Weaver F. M., Gross R. E., DeLong M. R., Arch. Neurol. 2011, 68, 165. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
