Enzymatically Bioactive Nucleus Pulposus Matrix Hydrogel Microspheres for Exogenous Stem Cells Therapy and Endogenous Repair Strategy to Achieve Disc Regeneration
- PMID: 38145353
- PMCID: PMC10933624
- DOI: 10.1002/advs.202304761
Enzymatically Bioactive Nucleus Pulposus Matrix Hydrogel Microspheres for Exogenous Stem Cells Therapy and Endogenous Repair Strategy to Achieve Disc Regeneration
Abstract
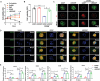
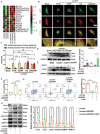
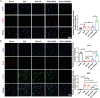
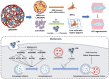
Exogenous stem cell therapy and endogenous repair has shown great potential in intervertebral disc regeneration. However, limited nutrients and accumulation of lactate largely impair the survival and regenerative capacity of implanted stem cells and endogenous nucleus pulposus cells (NPCs). Herein, an injectable hydrogel microsphere (LMGDNPs) have been developed by immersing lactate oxidase (LOX)-manganese dioxide (MnO2 ) nanozyme (LM) into glucose-enriched decellularized nucleus pulposus hydrogel microspheres (GDNPs) through a microfluidic system. LMGDNPs showed a delayed release profile of LOX and satisfactory enzymatic capacity in consuming lactate. Mesenchymal stem cells (MSCs) plated on LMGDNPs exhibited better cell viability than cells on GelMA and decellularized nucleus pulposus microspheres (DNP) and showed a obviously increased NPCs phenotype. LMGDNPs prevented MSCs and NPCs death and promoted extracellular matrix synthesis by exhausting lactate. It is determined that LMGDNPs promoted NPCs autophagy by activating transforming growth factor β2 overlapping transcript 1 (TGFB2-OT1), relying on the nanozyme. MSCs-loaded LMGDNPs largely preserved disc hydration and alleviated matrix degradation in vivo. Summarily, LMGDNPs promoted cell survival and matrix regeneration by providing a nutrient supply, exhausting lactate, and activating autophagy via TGFB2-OT1 and its downstream pathway and may serve as an ideal delivery system for exogenous stem cell therapy and endogenous repair.
Keywords: TGFB2-OT1; autophagy; intervertebral disc degeneration; lactate; microfluidic system; nanozyme.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
