Ammonium perchlorate: serum dosimetry, neurotoxicity, and resilience of the neonatal rat thyroid system
- PMID: 38145495
- PMCID: PMC11588387
- DOI: 10.1093/toxsci/kfad133
Ammonium perchlorate: serum dosimetry, neurotoxicity, and resilience of the neonatal rat thyroid system
Erratum in
-
Correction: Ammonium perchlorate: serum dosimetry, neurotoxicity, and resilience of the neonatal rat thyroid system.Toxicol Sci. 2024 Feb 28;198(1):155-156. doi: 10.1093/toxsci/kfae010. Toxicol Sci. 2024. PMID: 38308820 No abstract available.
Abstract
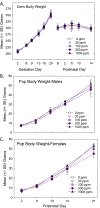
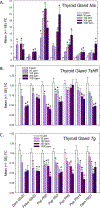
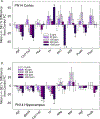
The environmental contaminant perchlorate impairs the synthesis of thyroid hormones by reducing iodine uptake into the thyroid gland. Despite this known action, moderate doses of perchlorate do not significantly alter serum thyroid hormone in rat pups born to exposed dams. We examined perchlorate dosimetry and responsivity of the thyroid gland and brain in offspring following maternal exposure to perchlorate. Pregnant rat dams were delivered perchlorate in drinking water (0, 30, 100, 300, 1000 ppm) from gestational day 6 to postnatal day (PN) 21. Perchlorate was present in the placenta, milk, and serum, the latter declining in pups over the course of lactation. Serum and brain thyroid hormone were reduced in pups at birth but recovered to control levels by PN2. Dramatic upregulation of Nis was observed in the thyroid gland of the exposed pup. Despite the return of serum thyroid hormone to control levels by PN2, expression of several TH-responsive genes was altered in the PN14 pup brain. Contextual fear learning was unimpaired in the adults, supporting previous reports. Declining levels of serum perchlorate and a profound upregulation of Nis gene expression in the thyroid gland are consistent with the rapid return to the euthyroid state in the neonate. However, despite this recovery, thyroid hormone insufficiencies in serum and brain beginning in utero and present at birth appear sufficient to alter TH action in the fetus and subsequent trajectory of brain development. Biomarkers of that altered trajectory remain in the brain of the neonate, demonstrating that perchlorate is not devoid of effects on the developing brain.
Keywords: AOP; brain; development; neurotoxicity; perchlorate; thyroid hormone.
Published by Oxford University Press on behalf of the Society of Toxicology 2023.
Figures





References
-
- Abt E, Spungen J, Pouillot R, Gamalo-Siebers M, Wirtz M. 2018. Update on dietary intake of perchlorate and iodine from u.S. Food and drug administration’s total diet study: 2008–2012. J Expo Sci Environ Epidemiol. 28(1):21–30. - PubMed
-
- Bernal J 2017. Thyroid hormone regulated genes in cerebral cortex development. The Journal of endocrinology. 232(2):R83–r97. - PubMed
-
- Blount BC, Valentin-Blasini L. 2006. Analysis of perchlorate, thiocyanate, nitrate and iodide in human amniotic fluid using ion chromatography and electrospray tandem mass spectrometry. Anal Chim Acta. 567(1):87–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

