Inhibition of porcine deltacoronavirus entry and replication by Cepharanthine
- PMID: 38145807
- PMCID: PMC10792575
- DOI: 10.1016/j.virusres.2023.199303
Inhibition of porcine deltacoronavirus entry and replication by Cepharanthine
Abstract
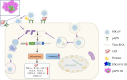
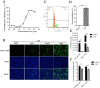
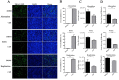
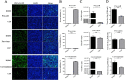
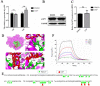
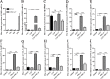
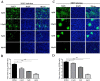
Porcine deltacoronavirus (PDCoV) is an emerging swine enteropathogenic coronavirus (CoV) that mainly causes acute diarrhea/vomiting, dehydration, and mortality in piglets, possessing economic losses and public health concerns. However, there are currently no proven effective antiviral agents against PDCoV. Cepharanthine (CEP) is a naturally occurring alkaloid used as a traditional remedy for radiation-induced symptoms, but its underlying mechanism of CEP against PDCoV has remained elusive. The aim of this study was to investigate the anti-PDCoV effects and mechanisms of CEP in LLC-PK1 cells. The results showed that the antiviral activity of CEP was based on direct action on cells, preventing the virus from attaching to host cells and virus replication. Importantly, Surface Plasmon Resonance (SPR) results showed that CEP has a moderate affinity to PDCoV receptor, porcine aminopeptidase N (pAPN) protein. AutoDock predicted that CEP can form hydrogen bonds with amino acid residues (R740, N783, and R790) in the binding regions of PDCoV and pAPN. In addition, RT-PCR results showed that CEP treatment could significantly reduce the transcription of ZBP1, cytokine (IL-1β and IFN-α) and chemokine genes (CCL-2, CCL-4, CCL-5, CXCL-2, CXCL-8, and CXCL-10) induced by PDCoV. Western blot analysis revealed that CEP could inhibit viral replication by inducing autophagy. In conclusion, our results suggest that the anti-PDCoV activity of CEP is not only relies on competing the virus binding with pAPN, but also affects the proliferation of the virus in vitro by downregulating the excessive immune response caused by the virus and inducing autophagy. CEP emerges as a promising candidate for potential anti-PDCoV therapeutic development.
Keywords: Antivirals; Cepharanthine; Entry; Porcine deltacoronavirus; Replication.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Porcine Deltacoronavirus Engages the Transmissible Gastroenteritis Virus Functional Receptor Porcine Aminopeptidase N for Infectious Cellular Entry.J Virol. 2018 May 29;92(12):e00318-18. doi: 10.1128/JVI.00318-18. Print 2018 Jun 15. J Virol. 2018. PMID: 29618640 Free PMC article.
-
Ergosterol peroxide exhibits antiviral and immunomodulatory abilities against porcine deltacoronavirus (PDCoV) via suppression of NF-κB and p38/MAPK signaling pathways in vitro.Int Immunopharmacol. 2021 Apr;93:107317. doi: 10.1016/j.intimp.2020.107317. Epub 2021 Jan 22. Int Immunopharmacol. 2021. PMID: 33493866 Free PMC article.
-
Contribution of porcine aminopeptidase N to porcine deltacoronavirus infection.Emerg Microbes Infect. 2018 Apr 11;7(1):65. doi: 10.1038/s41426-018-0068-3. Emerg Microbes Infect. 2018. PMID: 29636467 Free PMC article.
-
Porcine deltacoronavirus infection: Etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis.Virus Res. 2016 Dec 2;226:50-59. doi: 10.1016/j.virusres.2016.04.009. Epub 2016 Apr 13. Virus Res. 2016. PMID: 27086031 Free PMC article. Review.
-
Epidemiology, pathogenesis, immune evasion mechanism and vaccine development of porcine Deltacoronavirus.Funct Integr Genomics. 2024 Apr 24;24(3):79. doi: 10.1007/s10142-024-01346-7. Funct Integr Genomics. 2024. PMID: 38653845 Review.
Cited by
-
Antiviral Activity of 1-Deoxynojirimycin Extracts of Mulberry Leaves Against Porcine Epidemic Diarrhea Virus.Animals (Basel). 2025 Apr 23;15(9):1207. doi: 10.3390/ani15091207. Animals (Basel). 2025. PMID: 40362022 Free PMC article.
-
Insights into recent advancements in human and animal rotavirus vaccines: Exploring new frontiers.Virol Sin. 2025 Feb;40(1):1-14. doi: 10.1016/j.virs.2024.12.001. Epub 2024 Dec 11. Virol Sin. 2025. PMID: 39672271 Free PMC article. Review.
-
Advances research in porcine enteric coronavirus therapies and antiviral drugs.Vet Q. 2024 Dec;44(1):1-49. doi: 10.1080/01652176.2024.2421299. Epub 2024 Nov 1. Vet Q. 2024. PMID: 39484691 Free PMC article. Review.
-
Bisbenzylisoquinoline alkaloids inhibit influenza virus replication by disrupting endosomal acidification.Virol J. 2025 Jun 4;22(1):181. doi: 10.1186/s12985-025-02775-x. Virol J. 2025. PMID: 40468427 Free PMC article.
-
Genome-wide CRISPR/Cas9 library screen identifies C16orf62 as a host dependency factor for porcine deltacoronavirus infection.Emerg Microbes Infect. 2024 Dec;13(1):2400559. doi: 10.1080/22221751.2024.2400559. Epub 2024 Sep 13. Emerg Microbes Infect. 2024. PMID: 39222358 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

