Diffusion-Based 3D Bioprinting Strategies
- PMID: 38145962
- PMCID: PMC10885663
- DOI: 10.1002/advs.202306470
Diffusion-Based 3D Bioprinting Strategies
Abstract
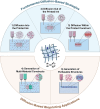
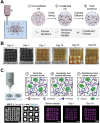
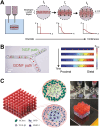
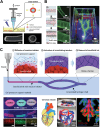
3D bioprinting has enabled the fabrication of tissue-mimetic constructs with freeform designs that include living cells. In the development of new bioprinting techniques, the controlled use of diffusion has become an emerging strategy to tailor the properties and geometry of printed constructs. Specifically, the diffusion of molecules with specialized functions, including crosslinkers, catalysts, growth factors, or viscosity-modulating agents, across the interface of printed constructs will directly affect material properties such as microstructure, stiffness, and biochemistry, all of which can impact cell phenotype. For example, diffusion-induced gelation is employed to generate constructs with multiple materials, dynamic mechanical properties, and perfusable geometries. In general, these diffusion-based bioprinting strategies can be categorized into those based on inward diffusion (i.e., into the printed ink from the surrounding air, solution, or support bath), outward diffusion (i.e., from the printed ink into the surroundings), or diffusion within the printed construct (i.e., from one zone to another). This review provides an overview of recent advances in diffusion-based bioprinting strategies, discusses emerging methods to characterize and predict diffusion in bioprinting, and highlights promising next steps in applying diffusion-based strategies to overcome current limitations in biofabrication.
Keywords: bioprinting; diffusion; interfacial gelation; multi-material constructs; perfusable structures.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Bernal P. N., Delrot P., Loterie D., Li Y., Malda J., Moser C., Levato R., Adv. Mater. 2019, 31, 1904209. - PubMed
-
- Größbacher G., Bartolf‐Kopp M., Gergely C., Bernal P. N., Florczak S., de Ruijter M., Rodriguez N. G., Groll J., Malda J., Jungst T., Levato R., Adv. Mater. 2023, 35, 2300756. - PubMed
-
- Murphy S. V., Atala A., Nat. Biotechnol. 2014, 32, 773. - PubMed
-
- Zhang Y. S., Haghiashtiani G., Hübscher T., Kelly D. J., Lee J. M., Lutolf M., McAlpine M. C., Yeong W. Y., Zenobi‐Wong M., Malda J., Nat. Rev. Methods Primers 2021, 1, 75.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
