Gastrodin alleviates premature senescence of vascular endothelial cells by enhancing the Nrf2/HO-1 signalling pathway
- PMID: 38146239
- PMCID: PMC10844697
- DOI: 10.1111/jcmm.18089
Gastrodin alleviates premature senescence of vascular endothelial cells by enhancing the Nrf2/HO-1 signalling pathway
Abstract
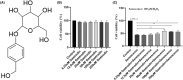
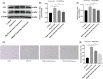
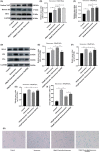
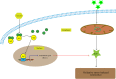
Endothelial dysfunction is an independent risk factor for stroke. The dysfunction of endothelial cells (EC) is closely concerned with EC senescence. Gastrodin (GAS) is an organic compound extracted from the dried root mass of the Orchidaceae plant Gastrodiae gastrodiae. It is used clinically to treat diseases such as vertebrobasilar insufficiency, vestibular neuronitis and vertigo. In the present study, we used hydrogen peroxide (H2 O2 )-induced human umbilical vein endothelial cells (HUVECs) to establish an in vitro EC senescence model and to investigate the role and mechanism of GAS in EC senescence. It's found that H2 O2 -treated HUVECs increased the proportion of senescence-associated β-galactosidase (SA β-gal) positive cells and the relative protein expression levels of senescence-associated cyclin p16 and p21. In addition, GAS reduced the proportion of SA β-gal positive cells and the relative protein expression levels of p16 and p21, and increased the proliferation and migration ability of HUVECs. Meanwhile, GAS increased the expression of the anti-oxidative stress protein HO-1 and its nuclear expression level of Nrf2. The anti-senescence effect of GAS was blocked when HO-1 expression was inhibited by SnPPIX. Furthermore, absence of HO-1 abolished the effect of GAS on HUVEC proliferation and migration. In conclusion, GAS ameliorated H2 O2 -induced cellular senescence and enhanced cell proliferation and migration by enhancing Nrf2/HO-1 signalling in HUVECs. These findings of our study expanded the understanding of GAS pharmacology and suggested that GAS may offer a potential therapeutic agent for stroke.
Keywords: Nrf2/HO-1; cell senescence; cerebrovascular disease; gastrodin; oxidative stress; proliferation.
© 2023 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Salicin prevents TNF-α-induced cellular senescence in human umbilical vein endothelial cells (HUVECs).Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):2618-2623. doi: 10.1080/21691401.2019.1629949. Artif Cells Nanomed Biotechnol. 2019. PMID: 31220953
-
Gastrodin prevents homocysteine-induced human umbilical vein endothelial cells injury via PI3K/Akt/eNOS and Nrf2/ARE pathway.J Cell Mol Med. 2021 Jan;25(1):345-357. doi: 10.1111/jcmm.16073. Epub 2020 Dec 15. J Cell Mol Med. 2021. PMID: 33320446 Free PMC article.
-
Dopamine D1 receptor activation ameliorates ox-LDL-induced endothelial cell senescence via CREB/Nrf2 pathway.Exp Cell Res. 2023 Apr 15;425(2):113542. doi: 10.1016/j.yexcr.2023.113542. Epub 2023 Mar 7. Exp Cell Res. 2023. PMID: 36894051
-
The Protective Effect of Chlorogenic Acid on Vascular Senescence via the Nrf2/HO-1 Pathway.Int J Mol Sci. 2020 Jun 25;21(12):4527. doi: 10.3390/ijms21124527. Int J Mol Sci. 2020. PMID: 32630570 Free PMC article.
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
Cited by
-
Gastrodin prevents myocardial injury in sleep-deprived mice by suppressing ferroptosis through SIRT6.Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):9111-9121. doi: 10.1007/s00210-024-03230-4. Epub 2024 Jun 19. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38896272
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

