Inhibition of lipid synthesis by the HIV integrase strand transfer inhibitor elvitegravir in primary rat oligodendrocyte cultures
- PMID: 38146334
- PMCID: PMC10749327
- DOI: 10.3389/fnmol.2023.1323431
Inhibition of lipid synthesis by the HIV integrase strand transfer inhibitor elvitegravir in primary rat oligodendrocyte cultures
Abstract
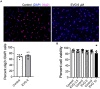
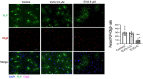
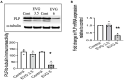
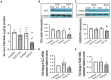
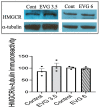
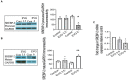
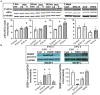
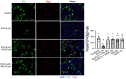
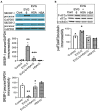
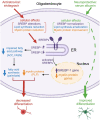
Combined antiretroviral therapy (cART) has greatly decreased mortality and morbidity among persons with HIV; however, neurologic impairments remain prevalent, in particular HIV-associated neurocognitive disorders (HANDs). White matter damage persists in cART-treated persons with HIV and may contribute to neurocognitive dysfunction as the lipid-rich myelin membrane of oligodendrocytes is essential for efficient nerve conduction. Because of the importance of lipids to proper myelination, we examined the regulation of lipid synthesis in oligodendrocyte cultures exposed to the integrase strand transfer inhibitor elvitegravir (EVG), which is administered to persons with HIV as part of their initial regimen. We show that protein levels of genes involved in the fatty acid pathway were reduced, which correlated with greatly diminished de novo levels of fatty acid synthesis. In addition, major regulators of cellular lipid metabolism, the sterol regulatory element-binding proteins (SREBP) 1 and 2, were strikingly altered following exposure to EVG. Impaired oligodendrocyte differentiation manifested as a marked reduction in mature oligodendrocytes. Interestingly, most of these deleterious effects could be prevented by adding serum albumin, a clinically approved neuroprotectant. These new findings, together with our previous study, strengthen the possibility that antiretroviral therapy, at least partially through lipid dysregulation, may contribute to the persistence of white matter changes observed in persons with HIV and that some antiretrovirals may be preferable as life-long therapy.
Keywords: HIV; Hand; SREBP; cART; myelin; oligodendrocyte.
Copyright © 2023 Monnerie, Romer, Roth, Long, Millar, Jordan-Sciutto and Grinspan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- AIDSinfo (2019) Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. https://aidsinfo.nih.gov/guidelines
-
- Borjabad A., Morgello S., Chao W., Kim S.-Y., Brooks A. I., Murray J., et al. . (2011). Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog. 7:e1002213. doi: 10.1371/journal.ppat.1002213, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

