NLRP6 controls pulmonary inflammation from cigarette smoke in a gut microbiota-dependent manner
- PMID: 38146368
- PMCID: PMC10749332
- DOI: 10.3389/fimmu.2023.1224383
NLRP6 controls pulmonary inflammation from cigarette smoke in a gut microbiota-dependent manner
Abstract
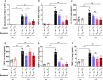
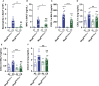
Chronic obstructive pulmonary disease (COPD) is a major health issue primarily caused by cigarette smoke (CS) and characterized by breathlessness and repeated airway inflammation. NLRP6 is a cytosolic innate receptor controlling intestinal inflammation and orchestrating the colonic host-microbial interface. However, its roles in the lungs remain largely unexplored. Using CS exposure models, our data show that airway inflammation is strongly impaired in Nlrp6-deficient mice with drastically fewer recruited neutrophils, a key cell subset in inflammation and COPD. We found that NLRP6 expression in lung epithelial cells is important to control airway and lung tissue inflammation in an inflammasome-dependent manner. Since gut-derived metabolites regulate NLRP6 inflammasome activation in intestinal epithelial cells, we investigated the link between NLRP6, CS-driven lung inflammation, and gut microbiota composition. We report that acute CS exposure alters gut microbiota in both wild-type (WT) and Nlrp6-deficient mice and that antibiotic treatment decreases CS-induced lung inflammation. In addition, gut microbiota transfer from dysbiotic Nlrp6-deficient mice to WT mice decreased airway lung inflammation in WT mice, highlighting an NLRP6-dependent gut-to-lung axis controlling pulmonary inflammation.
Keywords: NLRP6; cigarette smoke-exposure; gut microbiota; gut to lung axis; lung inflammation.
Copyright © 2023 Nascimento, Huot-Marchand, Fanny, Straube, Le Bert, Savigny, Apetoh, Van Snick, Trovero, Chamaillard, Quesniaux, Ryffel, Gosset, Gombault, Riteau, Sokol and Couillin.
Conflict of interest statement
Author FT was employed by company ArtImmunne SAS. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

