Comparative analysis of EZH2, p16 and p53 expression in uterine carcinosarcomas
- PMID: 38146588
- PMCID: PMC10749357
- DOI: 10.3389/pore.2023.1611547
Comparative analysis of EZH2, p16 and p53 expression in uterine carcinosarcomas
Abstract
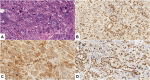
Introduction: The role of p16 and p53 immunohistochemistry in the diagnosis of rare and aggressive uterine carcinosarcoma (UCS) has been well established. However, enhancer of zeste homolog 2 (EZH2), a histone methyltransferase and a member of the polycomb group family is a relatively new biomarker, with limited published data on its significance in this tumor type. The goal of this study was to examine EZH2 expression in UCS and its components, in correlation with morphological features, and p16 and p53 staining patterns. Methods: Twenty-eight UCSs were included in the study. EZH2, p16 and p53 immunoreactivity were assessed independently by two pathologists in both tumor components (epithelial and mesenchymal). EZH2 and p16 immunostains were scored semiquantitatively: based on the percentage and intensity of tumor cell staining a binary staining index ("high- or low-expressing") was calculated. The p53 staining pattern was evaluated as wild-type or aberrant (diffuse nuclear, null, or cytoplasmic expression). Statistical tests were used to evaluate the correlation between staining patterns for all three markers and the different tumor components and histotypes. Results: High EZH2 and p16 expression and aberrant p53 patterns were present in 89.3% 78.6% and 85.7% of the epithelial component and in 78.6%, 62.5% and 82.1% of the mesenchymal component, respectively. Differences among these expression rates were not found to be significant (p > 0.05). Regarding the epithelial component, aberrant p53 pattern was found to be significantly (p = 0.0474) more frequent in the serous (100%) than in endometrioid (66.6%) histotypes. Within the mesenchymal component, p53 null expression pattern occurred significantly (p = 0.0257) more frequently in heterologous sarcoma components (71.4%) compared to the homologous histotype (18.8%). Conclusion: In conclusion, EZH2, p16 and p53 seem to play a universal role in the pathogenesis of UCS; however, a distinctive pattern of p53 expression appears to exist between the serous and endometrioid carcinoma components and also between the homologous and heterologous sarcoma components.
Keywords: EZH2; carcinosarcoma; p16; p53; uterine cancer.
Copyright © 2023 Makk, Bohonyi, Oszter, Éles, Tornóczky, Tóth, Kálmán and Kovács.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
PTEN immunohistochemical expression is suppressed in G1 endometrioid adenocarcinoma of the uterine corpus.J Cancer Res Clin Oncol. 2004 Mar;130(3):161-8. doi: 10.1007/s00432-003-0517-8. Epub 2003 Dec 20. J Cancer Res Clin Oncol. 2004. PMID: 14689303 Free PMC article.
-
Nuclear β-Catenin Expression in the Context of Abnormal p53 Expression Indicates a Nonserous Histotype in Endometrial Carcinoma.Int J Gynecol Pathol. 2023 Sep 1;42(5):435-442. doi: 10.1097/PGP.0000000000000923. Epub 2022 Oct 11. Int J Gynecol Pathol. 2023. PMID: 36731035
-
Correlative Assessment of p53 Immunostaining Patterns and TP53 Mutation Status by Next-Generation Sequencing in High-Grade Endometrial Carcinomas.Int J Gynecol Pathol. 2023 Nov 1;42(6):567-575. doi: 10.1097/PGP.0000000000000930. Epub 2022 Nov 15. Int J Gynecol Pathol. 2023. PMID: 36730675
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Tissue-based Immunohistochemical Biomarker Accuracy in the Diagnosis of Malignant Glandular Lesions of the Uterine Cervix: A Systematic Review of the Literature and Meta-Analysis.Int J Gynecol Pathol. 2017 Jul;36(4):310-322. doi: 10.1097/PGP.0000000000000345. Int J Gynecol Pathol. 2017. PMID: 27801764 Free PMC article.
Cited by
-
EZH2 expression in corneal and conjunctival intraepithelial neoplasia and squamous cell carcinoma: a potential marker for diagnosis.Int Ophthalmol. 2025 Jun 1;45(1):219. doi: 10.1007/s10792-025-03595-2. Int Ophthalmol. 2025. PMID: 40451935
References
-
- Loda M, Mucci LA, Mittelstadt ML, Hemelrijck MV, Cotter MB. Pathology and epidemiology of cancer. Cham: Springer International Publishing; (2016). 10.1007/978-3-319-35153-7 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

