New cyclophilin D inhibitor rescues mitochondrial and cognitive function in Alzheimer's disease
- PMID: 38146639
- PMCID: PMC11484516
- DOI: 10.1093/brain/awad432
New cyclophilin D inhibitor rescues mitochondrial and cognitive function in Alzheimer's disease
Abstract
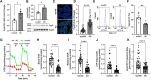
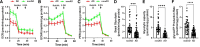
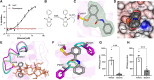
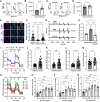
Mitochondrial dysfunction is an early pathological feature of Alzheimer disease and plays a crucial role in the development and progression of Alzheimer's disease. Strategies to rescue mitochondrial function and cognition remain to be explored. Cyclophilin D (CypD), the peptidylprolyl isomerase F (PPIase), is a key component in opening the mitochondrial membrane permeability transition pore, leading to mitochondrial dysfunction and cell death. Blocking membrane permeability transition pore opening by inhibiting CypD activity is a promising therapeutic approach for Alzheimer's disease. However, there is currently no effective CypD inhibitor for Alzheimer's disease, with previous candidates demonstrating high toxicity, poor ability to cross the blood-brain barrier, compromised biocompatibility and low selectivity. Here, we report a new class of non-toxic and biocompatible CypD inhibitor, ebselen, using a conventional PPIase assay to screen a library of ∼2000 FDA-approved drugs with crystallographic analysis of the CypD-ebselen crystal structure (PDB code: 8EJX). More importantly, we assessed the effects of genetic and pharmacological blockade of CypD on Alzheimer's disease mitochondrial and glycolytic bioenergetics in Alzheimer's disease-derived mitochondrial cybrid cells, an ex vivo human sporadic Alzheimer's disease mitochondrial model, and on synaptic function, inflammatory response and learning and memory in Alzheimer's disease mouse models. Inhibition of CypD by ebselen protects against sporadic Alzheimer's disease- and amyloid-β-induced mitochondrial and glycolytic perturbation, synaptic and cognitive dysfunction, together with suppressing neuroinflammation in the brain of Alzheimer's disease mouse models, which is linked to CypD-related membrane permeability transition pore formation. Thus, CypD inhibitors have the potential to slow the progression of neurodegenerative diseases, including Alzheimer's disease, by boosting mitochondrial bioenergetics and improving synaptic and cognitive function.
Keywords: Alzheimer disease; CypD inhibitor; amyloid beta; high-throughput screening; mitochondrial respiratory and glycolytic function.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures




References
-
- Ray WJ, Buggia-Prevot V. Novel targets for Alzheimer's disease: A view beyond amyloid. Annu Rev Med. 2021;72:15–28. - PubMed
-
- Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105(8-9):855–870. - PubMed
-
- Humphries KM, Yoo Y, Szweda LI. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry. 1998;37:552–557. - PubMed

