Event-free survival of maralixibat-treated patients with Alagille syndrome compared to a real-world cohort from GALA
- PMID: 38146932
- PMCID: PMC11095900
- DOI: 10.1097/HEP.0000000000000727
Event-free survival of maralixibat-treated patients with Alagille syndrome compared to a real-world cohort from GALA
Abstract
Background and aims: Alagille syndrome (ALGS) is characterized by chronic cholestasis with associated pruritus and extrahepatic anomalies. Maralixibat, an ileal bile acid transporter inhibitor, is an approved pharmacologic therapy for cholestatic pruritus in ALGS. Since long-term placebo-controlled studies are not feasible or ethical in children with rare diseases, a novel approach was taken comparing 6-year outcomes from maralixibat trials with an aligned and harmonized natural history cohort from the G lobal AL agille A lliance (GALA) study.
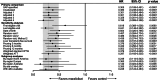
Approach and results: Maralixibat trials comprise 84 patients with ALGS with up to 6 years of treatment. GALA contains retrospective data from 1438 participants. GALA was filtered to align with key maralixibat eligibility criteria, yielding 469 participants. Serum bile acids could not be included in the GALA filtering criteria as these are not routinely performed in clinical practice. Index time was determined through maximum likelihood estimation in an effort to align the disease severity between the two cohorts with the initiation of maralixibat. Event-free survival, defined as the time to first event of manifestations of portal hypertension (variceal bleeding, ascites requiring therapy), surgical biliary diversion, liver transplant, or death, was analyzed by Cox proportional hazards methods. Sensitivity analyses and adjustments for covariates were applied. Age, total bilirubin, gamma-glutamyl transferase, and alanine aminotransferase were balanced between groups with no statistical differences. Event-free survival in the maralixibat cohort was significantly better than the GALA cohort (HR, 0.305; 95% CI, 0.189-0.491; p <0.0001). Multiple sensitivity and subgroup analyses (including serum bile acid availability) showed similar findings.
Conclusions: This study demonstrates a novel application of a robust statistical method to evaluate outcomes in long-term intervention studies where placebo comparisons are not feasible, providing wide application for rare diseases. This comparison with real-world natural history data suggests that maralixibat improves event-free survival in patients with ALGS.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Bettina E. Hansen consults and received grants from Calliditas, CymaBay, and Intercept. She consults for Albireo, Enyo, Eiger, Ipsen, and Mirum. Shannon M. Vandriel consults and received grants from Mirum. She consults for Albireo. Pamela Vig is employed by and owns stock in Mirum. Will Garner is employed by and owns stock in Mirum. Douglas B. Mogul is employed by and owns stock in Mirum. Kathleen M. Loomes consults and received grants from Albireo and Mirum. She consults for Travere. Dorota Gliwicz-Miedzińska received grants from ExCEEd Orphan. Emmanuel M. Gonzales consults, advises, is on the speakers’ bureau, and received grants from Albireo and Mirum. He consults and is on the speakers’ bureau for CTRS. He consults for Vivet. Emmanuel Jacquemin consults for CTRS and Vivet. Lorenzo D’Antiga consults and advises Albireo and Mirum. He consults for Advanz, Spark, and Tome. He advises Alexion, AstraZeneca, Genespire, Selecta, and Vivet. Emanuele Nicastro consults for Albireo and Mirum. Henrik Arnell advises Mirum. He is employed by Albireo and Baxter. Étienne Sokal consults, advises, owns stock, holds intellectual property rights, and has other interests with Cellaion. He consults for Ipsen and Mirum. Saul J. Karpen consults for Albireo, HemoShear, Intercept, and Mirum. Rene Romero consults for Albireo and Mirum. He received grants from Gilead. Noelle H. Ebel consults and is on the speakers’ bureau for Ipsen and Mirum. Shikha S. Sundaram advises Albireo and Mirum. Ryan T. Fischer advises and is on the speakers’ bureau for Albireo, Ipsen, and Mirum. Henry C. Lin advises Albireo and Mirum. M. Kyle Jensen consults, advises, and received grants from Albireo. He consults for GLG and Guidepoint Global. Palaniswamy Karthikeyan consults for Cepton Strategies. He advises Albireo. Giuseppe Indolfi advises Albireo and Mirum. Henkjan J. Verkade consults and advises Albireo and Mirum. Pamela L. Valentino is on the speakers’ bureau for Mirum. Antal Dezsőfi advises Mirum. Kathleen Schwarz consults and advises Sarepta. She consults for Mirum and Up to Date. She advises and received grants from Albireo and Gilead. She is employed by NASPGHAN. Pier Luigi Calvo advises Albireo, Ipsen, Mirum, and Nestlé. Andréanne N. Zizzo advises Mirum. Gabriella Nebbia consults and advises Albireo. Rima Fawaz consults for Albireo, the Hepatic Adjudication Committee, and Mirum. Wikrom Karnsakul received grants from Albireo, Gilead, Mirum, and Travere. Cristina Molera Busoms advises Alexion, Mirum, and Orphalan. Deirdre A. Kelly consults, advises, received grants, and is employed by Albireo and Mirum. She consults, advises, and received grants from Intercept. She consults and advises AstraZeneca. She consults for Alnylam, Freeline, GlaxoSmithKline, Orphalan, and Takeda. She received grants from AbbVie and Gilead. Jesus M. Banales consults, advises, and received grants from Albireo and CymaBay. He consults and advises Ikan Biotech and OWL-Rubio Metabolomics. He is on the speakers’ bureau and received grants from Incyte. He is on the speakers’ bureau for AstraZeneca and Intercept. He received grants from Roche. Quais Mujawar advises Medison and Mirum. He owns stock in Bausch Health and Sun Pharma. Orith Waisbourd-Zinman consults for Neopharm Israel. Sabina Wiecek is employed by the Department of Pediatrics, Medical University of Silesia. Raquel Borges Pinto is employed by Hospital Criança Conceição. Nanda Kerkar advises Albireo, Alexion, Ipsen, and Mirum. She received royalties from Elsevier. Jernej Brecelj is on the speakers’ bureau for Swixx Biopharma. Eberhard Lurz consults, advises, and is on the speakers’ bureau for Ipsen, Mirum, Nutricia, and Takeda. Richard J. Thompson consults and owns stock in Generation Bio and Rectify Pharma. He consults for Albireo, Alnylam, and Mirum. Binita M. Kamath consults and received grants from Albireo and Mirum. She consults for Astellas. The remaining authors have no conflicts to report.
Figures




References
-
- Bergasa NV. Pruritus of cholestasis In: Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014:61–88.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

