The Japanese Breast Cancer Society clinical practice guidelines for epidemiology and prevention of breast cancer, 2022 edition
- PMID: 38147174
- PMCID: PMC10902093
- DOI: 10.1007/s12282-023-01531-9
The Japanese Breast Cancer Society clinical practice guidelines for epidemiology and prevention of breast cancer, 2022 edition
Abstract
The Japanese Breast Cancer Society Clinical Practice Guidelines for Epidemiology and Prevention of Breast Cancer, 2022 Edition.
Keywords: 2022; Breast; Epidemiology; Guideline; JBCS; Prevention.
© 2023. The Author(s).
Conflict of interest statement
MK received honoraria for lectures from AstraZeneca, Chugai, Eisai, Eli Lilly and Company, Kyowa-Kirin, MSD, Novartis, and Pfizer; SO received honoraria for lectures from AstraZeneca, Chugai, Eizai and Eli Lilly; KT received honoraria for lectures from ASKA Pharmaceutical Co. Ltd., Bayer Yakuhin, Ltd., Fuji Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Tsumura & Co; YY received research funding from Chugai, Daiichi-Sankyo, Eisai, Kyowa-Kirin, Lilly, Nippon-Kayaku, Novartis, Pfizer, Taiho and Takeda; YY received honoraria for lectures from AstraZeneca, Chugai, Daiichi-Sankyo, Eisai, Exact Science, Kyowa-Kirin, Lilly, MSD, Nippon-Kayaku, Novartis, Pfizer, Sysmex, Taiho and Takeda; YY participated on advisory boards for AstraZeneca, Chugai, Daiichi-Sankyo, Lilly, MSD, Novartis and Pfizer; YY was a member of the board of directors of the Japanese Breast Cancer Society and Japan Breast Cancer Research Group; HI received grants from AstraZeneca, Chugai and Daiichi-Sankyo; HI received consulting fees from AstraZeneca, Chugai, Daiichi-Sankyo, Gilead, Lilly, MSD and Pfizer; HI received honoraria from AstraZeneca, Chugai, Daiichi-Sankyo, Kyowa Kirin, Lilly, MSD, Pfizer and Taiho; SS received grants from AstraZeneca, Chugai, Daiichi-Sankyo, Eisai, MSD and Taiho and Takeda; SS received honoraria from AstraZeneca, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Kyowa Kirin, MSD, Nipponkayaku, Novartis, Ono, Pfizer, Taiho and Takeda; SS participated on advisory boards for AstraZeneca, Chugai/Roche, Daiichi-Sankyo, Eli Lilly, Kyowa Kirin, MSD and Pfizer; SS was a member of the board of directors of Breast International Group (BIG), Japan Breast Cancer Research Group (JBCRG), Japanese Breast Cancer Society (JBCS) and Japanese Society of Medical Oncology (JSMO); MI, SY, HO, MA, TN, SO, KS, AA, FM, CY have no conflict of interest.
Figures


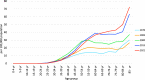

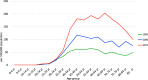
References
-
- Minds (Medical Information Distribution Service) Manual for Clinical Practice Guideline Development 2020 ver. 3.0. 2020.
-
- The Japanese Breast Cancer Society clinical practice guideline for treatment of breast cancer, 2022 edition. The Japanese Breast Cancer Society Clinical Practice Guideline. Kanehara & Co., Ltd: Tokyo; 2022.
-
- The Japanese Breast Cancer Society clinical practice guideline for epidemiology and diagnosis of breast cancer, 2022 edition. The Japanese Breast Cancer Society Clinical Practice Guideline. Kanehara & Co., Ltd: Tokyo; 2022.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

