VFMAP predicted hepatocellular carcinoma development in patients with chronic hepatitis C who were treated with direct-acting antiviral and achieved sustained virologic response
- PMID: 38147196
- PMCID: PMC11972991
- DOI: 10.1007/s10396-023-01398-5
VFMAP predicted hepatocellular carcinoma development in patients with chronic hepatitis C who were treated with direct-acting antiviral and achieved sustained virologic response
Erratum in
-
Correction: VFMAP predicted hepatocellular carcinoma development in patients with chronic hepatitis C who were treated with direct-acting antiviral and achieved sustained virologic response.J Med Ultrason (2001). 2025 Jul;52(3):351. doi: 10.1007/s10396-025-01537-0. J Med Ultrason (2001). 2025. PMID: 40188408 Free PMC article. No abstract available.
Abstract
Purpose: Risk factors for the development of hepatocellular carcinoma (HCC) remain unclear in patients with hepatitis C virus (HCV) who achieve sustained virological response (SVR) after direct-acting antiviral (DAA) therapy. This study investigated the usefulness of the VFMAP scoring system for predicting the development of HCC in these patients.
Methods: This study included 358 patients with HCV who achieved SVR after DAA treatment. The VFMAP system defines and scores cutoff values for virtual touch quantification (VTQ), fasting plasma glucose, sex, age, and alpha-fetoprotein values. All patients were grouped according to their VFMAP scores as follows: 0 or 1 point, low-score group; 2 or 3 points, intermediate-score group; and 4 or 5 points, high-score group.
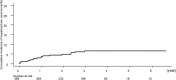
Results: Nineteen patients developed HCC. The median follow-up duration was 3.2 (1.5-4.0) years. The respective cumulative incidence rates of HCC at 12, 24, and 36 months were as follows in different subgroups: all study patients, 3.0%, 4.8%, and 6.6%; low-score group, 0.96%, 0.96%, and 0.96%; intermediate-score group, 2.6%, 4.5%, and 6.8%; and high-score group, 10.0%, 15.3%, and 18.5%. The cumulative incidence rates of HCC in the high-score group were significantly higher than those in the low- and intermediate-score groups (p < 0.001 and < 0.05, respectively).
Conclusion: VFMAP accurately predicted the development of HCC in HCV patients who achieved SVR following treatment with DAAs.
Keywords: Direct-acting antiviral; Hepatocellular carcinoma; Sustained virological response; VFMAP scoring system.
© 2023. The Author(s), under exclusive licence to The Japan Society of Ultrasonics in Medicine.
Conflict of interest statement
Toshifumi Tada: Lecture fees from AbbVie, Chugai Pharmaceutical, and Eisai. Takashi Nishimura and Hiroko Iijima: Lecture fees from Otsuka Pharmaceutical and research funding from Canon Medical Systems and GE Healthcare Japan. None of the other authors have potential conflicts of interest to declare.
Figures

References
-
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–76. - PubMed
-
- European Association for the Study of the Liver, Clinical Practice Guidelines Panel: Chair, EASL Governing Board representative: Panel members. EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73:1170–218. - PubMed
-
- Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with directacting antiviral agents. Gastroenterology. 2017;153:996–1005. - PubMed
-
- Nahon P, Layese R, Bourcier V, et al. ANRS CO12 CirVir Group Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155:1436–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

