FcγRIIB Is an Immune Checkpoint Limiting the Activity of Treg-Targeting Antibodies in the Tumor Microenvironment
- PMID: 38147316
- PMCID: PMC10911703
- DOI: 10.1158/2326-6066.CIR-23-0389
FcγRIIB Is an Immune Checkpoint Limiting the Activity of Treg-Targeting Antibodies in the Tumor Microenvironment
Abstract
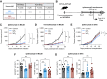
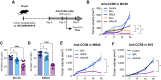
Preclinical murine data indicate that fragment crystallizable (Fc)-dependent depletion of intratumoral regulatory T cells (Treg) is a major mechanism of action of anti-CTLA-4. However, the two main antibodies administered to patients (ipilimumab and tremelimumab) do not recapitulate these effects. Here, we investigate the underlying mechanisms responsible for the limited Treg depletion observed with these therapies. Using an immunocompetent murine model humanized for CTLA-4 and Fcγ receptors (FcγR), we show that ipilimumab and tremelimumab exhibit limited Treg depletion in tumors. Immune profiling of the tumor microenvironment (TME) in both humanized mice and humans revealed high expression of the inhibitory Fc receptor, FcγRIIB, which limits antibody-dependent cellular cytotoxicity/phagocytosis. Blocking FcγRIIB in humanized mice rescued the Treg-depleting capacity and antitumor activity of ipilimumab. Furthermore, Fc engineering of antibodies targeting Treg-associated targets (CTLA-4 or CCR8) to minimize FcγRIIB binding significantly enhanced Treg depletion, resulting in increased antitumor activity across various tumor models. Our results define the inhibitory FcγRIIB as an immune checkpoint limiting antibody-mediated Treg depletion in the TME, and demonstrate Fc engineering as an effective strategy to overcome this limitation and improve the efficacy of Treg-targeting antibodies.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures



Update of
-
FcyRIIB is a novel immune checkpoint in the tumor microenvironment limiting activity of Treg-targeting antibodies.bioRxiv [Preprint]. 2023 Jan 20:2023.01.19.522856. doi: 10.1101/2023.01.19.522856. bioRxiv. 2023. Update in: Cancer Immunol Res. 2024 Mar 4;12(3):322-333. doi: 10.1158/2326-6066.CIR-23-0389. PMID: 36711504 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

