Implementation of Recommendations on the Use of Corticosteroids in Severe COVID-19
- PMID: 38147336
- PMCID: PMC10751594
- DOI: 10.1001/jamanetworkopen.2023.46502
Implementation of Recommendations on the Use of Corticosteroids in Severe COVID-19
Abstract
Importance: Research diversity and representativeness are paramount in building trust, generating valid biomedical knowledge, and possibly in implementing clinical guidelines.
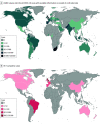
Objectives: To compare variations over time and across World Health Organization (WHO) geographic regions of corticosteroid use for treatment of severe COVID-19; secondary objectives were to evaluate the association between the timing of publication of the RECOVERY (Randomised Evaluation of COVID-19 Therapy) trial (June 2020) and the WHO guidelines for corticosteroids (September 2020) and the temporal trends observed in corticosteroid use by region and to describe the geographic distribution of the recruitment in clinical trials that informed the WHO recommendation.
Design, setting, and participants: This prospective cohort study of 434 851 patients was conducted between January 31, 2020, and September 2, 2022, in 63 countries worldwide. The data were collected under the auspices of the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC)-WHO Clinical Characterisation Protocol for Severe Emerging Infections. Analyses were restricted to patients hospitalized for severe COVID-19 (a subset of the ISARIC data set).
Exposure: Corticosteroid use as reported to the ISARIC-WHO Clinical Characterisation Protocol for Severe Emerging Infections.
Main outcomes and measures: Number and percentage of patients hospitalized with severe COVID-19 who received corticosteroids by time period and by WHO geographic region.
Results: Among 434 851 patients with confirmed severe or critical COVID-19 for whom receipt of corticosteroids could be ascertained (median [IQR] age, 61.0 [48.0-74.0] years; 53.0% male), 174 307 (40.1%) received corticosteroids during the study period. Of the participants in clinical trials that informed the guideline, 91.6% were recruited from the United Kingdom. In all regions, corticosteroid use for severe COVID-19 increased, but this increase corresponded to the timing of the RECOVERY trial (time-interruption coefficient 1.0 [95% CI, 0.9-1.2]) and WHO guideline (time-interruption coefficient 1.9 [95% CI, 1.7-2.0]) publications only in Europe. At the end of the study period, corticosteroid use for treatment of severe COVID-19 was highest in the Americas (5421 of 6095 [88.9%]; 95% CI, 87.7-90.2) and lowest in Africa (31 588 of 185 191 [17.1%]; 95% CI, 16.8-17.3).
Conclusions and relevance: The results of this cohort study showed that implementation of the guidelines for use of corticosteroids in the treatment of severe COVID-19 varied geographically. Uptake of corticosteroid treatment was lower in regions with limited clinical trial involvement. Improving research diversity and representativeness may facilitate timely knowledge uptake and guideline implementation.
Conflict of interest statement
Figures