Prenatal Exposure to Poly- and Perfluoroalkyl Substances (2009-2014) and Vaccine Antibody Titers of Measles, Mumps, Rubella, and Varicella in Children Four to Eight Years Old from the Healthy Start Cohort
- PMID: 38147368
- PMCID: PMC10750888
- DOI: 10.1289/EHP12863
Prenatal Exposure to Poly- and Perfluoroalkyl Substances (2009-2014) and Vaccine Antibody Titers of Measles, Mumps, Rubella, and Varicella in Children Four to Eight Years Old from the Healthy Start Cohort
Abstract
Background: Prenatal exposures to certain poly- and perfluoroalkyl substances (PFAS) are associated with reduced humoral responses to some childhood immunizations.
Objective: We estimated associations between prenatal PFAS exposure and child antibody titers for measles, mumps, rubella (MMR), and varicella after immunization.
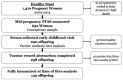
Methods: We measured serum antibody titers of 145 children (4-8 y old) enrolled in the Healthy Start cohort in Colorado, whose mothers had PFAS quantified mid-pregnancy (2009-2014). We used linear and logistic regression models to assess the relationship between five PFAS detected in of mothers and continuous or non-high-censored ("low") antibody titers and quantile g-computation to evaluate the overall effect of the PFAS mixture.
Results: Median concentrations of individual PFAS were at or below the median reported among females in the United States. After receiving two vaccine doses, seropositive levels of antibodies were detected among most (93%-100%) children. Each log-unit increase in perfluorononanoate was associated with 2.09 [95% confidence interval (CI): 1.13, 3.87] times higher odds of a low measles titer, and each log-unit increase in perfluorooctanoate was associated with 2.46 (95% CI: 1.28, 4.75) times higher odds of a low mumps titer. Odds ratios for all other PFAS were elevated, but CIs included the null. Each quartile increase in the PFAS mixture was associated with 1.35 (95% CI: 0.80, 2.26) times higher odds of a low measles titer and 1.44 (95% CI: 0.78, 2.64) times higher odds of a low mumps titer. No significant associations were observed between PFAS and varicella or rubella antibodies. In stratified analyses, associations were negative among female children, except for perfluorohexane sulfonate and varicella, whereas they were positive among males.
Discussion: Some prenatal PFAS were associated with lower antibody titers among fully immunized children. The potential for immunotoxic effects of PFAS requires further investigation in a larger study, because exposure is ubiquitous globally. https://doi.org/10.1289/EHP12863.
Figures


References
-
- Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, et al. 2013. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol 10(4):373–379, PMID: , 10.3109/1547691X.2012.755580. - DOI - PubMed
-
- Dalsager L, Christensen N, Husby S, Kyhl H, Nielsen F, Høst A, et al. 2016. Association between prenatal exposure to perfluorinated compounds and symptoms of infections at age 1-4 years among 359 children in the Odense Child Cohort. Environ Int 96:58–64, PMID: , 10.1016/j.envint.2016.08.026. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
