Social anxiety disorder-associated gut microbiota increases social fear
- PMID: 38147649
- PMCID: PMC10769841
- DOI: 10.1073/pnas.2308706120
Social anxiety disorder-associated gut microbiota increases social fear
Erratum in
-
Correction for Ritz et al., Social anxiety disorder-associated gut microbiota increases social fear.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2424381121. doi: 10.1073/pnas.2424381121. Epub 2024 Dec 20. Proc Natl Acad Sci U S A. 2025. PMID: 39705317 Free PMC article. No abstract available.
Abstract
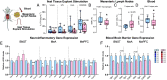
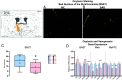
Social anxiety disorder (SAD) is a crippling psychiatric disorder characterized by intense fear or anxiety in social situations and their avoidance. However, the underlying biology of SAD is unclear and better treatments are needed. Recently, the gut microbiota has emerged as a key regulator of both brain and behaviour, especially those related to social function. Moreover, increasing data supports a role for immune function and oxytocin signalling in social responses. To investigate whether the gut microbiota plays a causal role in modulating behaviours relevant to SAD, we transplanted the microbiota from SAD patients, which was identified by 16S rRNA sequencing to be of a differential composition compared to healthy controls, to mice. Although the mice that received the SAD microbiota had normal behaviours across a battery of tests designed to assess depression and general anxiety-like behaviours, they had a specific heightened sensitivity to social fear, a model of SAD. This distinct heightened social fear response was coupled with changes in central and peripheral immune function and oxytocin expression in the bed nucleus of the stria terminalis. This work demonstrates an interkingdom basis for social fear responses and posits the microbiome as a potential therapeutic target for SAD.
Keywords: faecal transplant; microbiome; microbiota-gut-brain axis; social phobia.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Stein M. B., Kean Y. M., Disability and quality of life in social phobia: Epidemiologic findings. Am. J. Psychiatry 157, 1606–1613 (2000). - PubMed
-
- Keller M., The lifelong course of social anxiety disorder: A clinical perspective. Acta Psychiatr. Scand. 108, 85–94 (2003). - PubMed
-
- Baldwin D. S., et al. , Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: A revision of the 2005 guidelines from the British Association for Psychopharmacology. J. Psychopharmacol. 28, 403–439 (2014). - PubMed
-
- Patel A., Knapp M., Henderson J., Baldwin D., The economic consequences of social phobia. J. Affect. Disord. 68, 221–233 (2002). - PubMed
-
- Stein M. B., Stein D. J., Social anxiety disorder. The Lancet 371, 1115–1125 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

