Effects of Corticosterone on Beta-Amyloid-Induced Cell Death in SH-SY5Y Cells
- PMID: 38148553
- PMCID: PMC10762270
- DOI: 10.4062/biomolther.2023.133
Effects of Corticosterone on Beta-Amyloid-Induced Cell Death in SH-SY5Y Cells
Abstract
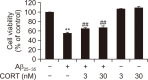
Alzheimer's disease (AD) is a neurodegenerative disease characterized by neuronal cell death and memory impairment. Corticosterone (CORT) is a glucocorticoid hormone produced by the hypothalamic-pituitary-adrenal axis in response to a stressful condition. Excessive stress and high CORT levels are known to cause neurotoxicity and aggravate various diseases, whereas mild stress and low CORT levels exert beneficial actions under pathophysiological conditions. However, the effects of mild stress on AD have not been clearly elucidated yet. In this study, the effects of low (3 and 30 nM) CORT concentration on Aβ25-35-induced neurotoxicity in SH-SY5Y cells and underlying molecular mechanisms have been investigated. Cytotoxicity caused by Aβ25-35 was significantly inhibited by the low concentration of CORT treatment in the cells. Furthermore, CORT pretreatment significantly reduced Aβ25-35-mediated pro-apoptotic signals, such as increased Bim/Bcl-2 ratio and caspase-3 cleavage. Moreover, low concentration of CORT treatment inhibited the Aβ25-35-induced cyclooxygenase-2 and pro-inflammatory cytokine expressions, including tumor necrosis factor-α and interleukin-1β. Aβ25-35 resulted in intracellular accumulation of reactive oxygen species and lipid peroxidation, which were effectively reduced by the low CORT concentration. As a molecular mechanism, low CORT concentration activated the nuclear factor-erythroid 2-related factor 2, a redox-sensitive transcription factor mediating cellular defense and upregulating the expression of antioxidant enzymes, such as NAD(P)H:quinone oxidoreductase, glutamylcysteine synthetase, and manganese superoxide dismutase. These findings suggest that low CORT concentration exerts protective actions against Aβ25-35-induced neurotoxicity and might be used to treat and/or prevent AD.
Keywords: Alzheimer’s disease; Beta-amyloid; Corticosterone; Inflammation; Neurotoxicity; Oxidative stress.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
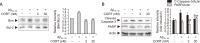
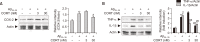

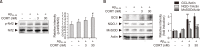

References
-
- Babić R., Babić M., Rastović P., Ćurlin M., Šimić J., Mandić K., Pavlović K. Resilience in health and illness. Psychiatr. Danub. 2020;32:226–232. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

