Doxorubicin Attenuates Free Fatty Acid-Induced Lipid Accumulation via Stimulation of p53 in HepG2 Cells
- PMID: 38148555
- PMCID: PMC10762281
- DOI: 10.4062/biomolther.2023.200
Doxorubicin Attenuates Free Fatty Acid-Induced Lipid Accumulation via Stimulation of p53 in HepG2 Cells
Abstract
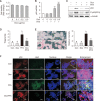
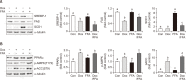
Non-alcoholic fatty liver disease (NAFLD) is characterized by excessive accumulation of fat in the liver, and there is a global increase in its incidence owing to changes in lifestyle and diet. Recent findings suggest that p53 is involved in the development of non-alcoholic fatty liver disease; however, the association between p53 expression and the disease remains unclear. Doxorubicin, an anticancer agent, increases the expression of p53. Therefore, this study aimed to investigate the role of doxorubicin-induced p53 upregulation in free fatty acid (FFA)-induced intracellular lipid accumulation. HepG2 cells were pretreated with 0.5 μg/mL of doxorubicin for 12 h, followed by treatment with FFA (0.5 mM) for 24 h to induce steatosis. Doxorubicin pretreatment upregulated p53 expression and downregulated the expression of endoplasmic reticulum stress- and lipid synthesis-associated genes in the FFA -treated HepG2 cells. Additionally, doxorubicin treatment upregulated the expression of AMP-activated protein kinase, a key modulator of lipid metabolism. Notably, siRNA-targeted p53 knockdown reversed the effects of doxorubicin in HepG2 cells. Moreover, doxorubicin treatment suppressed FFA -induced lipid accumulation in HepG2 spheroids. Conclusively, these results suggest that doxorubicin possesses potential application for the regulation of lipid metabolism by enhance the expression of p53 an in vitro NAFLD model.
Keywords: AMP-activated protein kinase; Doxorubicin; Fatty acid synthesis; Lipid metabolism; Non-alcoholic fatty liver disease; p53.
Figures
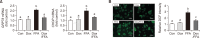



Similar articles
-
Lycopus lucidus Turcz. ex Benth. Attenuates free fatty acid-induced steatosis in HepG2 cells and non-alcoholic fatty liver disease in high-fat diet-induced obese mice.Phytomedicine. 2019 Mar 1;55:14-22. doi: 10.1016/j.phymed.2018.07.008. Epub 2018 Jul 18. Phytomedicine. 2019. PMID: 30668424
-
Lonicera caerulea Extract Attenuates Non-Alcoholic Fatty Liver Disease in Free Fatty Acid-Induced HepG2 Hepatocytes and in High Fat Diet-Fed Mice.Nutrients. 2019 Feb 26;11(3):494. doi: 10.3390/nu11030494. Nutrients. 2019. PMID: 30813654 Free PMC article.
-
Poria cocus Wolf Extract Ameliorates Hepatic Steatosis through Regulation of Lipid Metabolism, Inhibition of ER Stress, and Activation of Autophagy via AMPK Activation.Int J Mol Sci. 2019 Sep 27;20(19):4801. doi: 10.3390/ijms20194801. Int J Mol Sci. 2019. PMID: 31569635 Free PMC article.
-
Effect of Isoquercitrin on Free Fatty Acid-Induced Lipid Accumulation in HepG2 Cells.Molecules. 2023 Feb 3;28(3):1476. doi: 10.3390/molecules28031476. Molecules. 2023. PMID: 36771140 Free PMC article.
-
Kangtaizhi Granule Alleviated Nonalcoholic Fatty Liver Disease in High-Fat Diet-Fed Rats and HepG2 Cells via AMPK/mTOR Signaling Pathway.J Immunol Res. 2020 Aug 20;2020:3413186. doi: 10.1155/2020/3413186. eCollection 2020. J Immunol Res. 2020. PMID: 32884949 Free PMC article.
References
-
- Almanza A., Carlesso A., Chintha C., Creedican S., Doultsinos D., Leuzzi B., Luís A., McCarthy N., Montibeller L., More S., Papaioannou A., Püschel F., Sassano M. L., Skoko J., Agostinis P., de Belleroche J., Eriksson L. A., Fulda S., Gorman A. M., Healy S., Kozlov A., Muñoz-Pinedo C., Rehm M., Chevet E., Samali A. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. - DOI - PMC - PubMed
-
- Bravo R., Parra V., Gatica D., Rodriguez A. E., Torrealba N., Paredes F., Wang Z. V., Zorzano A., Hill J. A., Jaimovich E., Quest A. F., Lavandero S. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 2013;301:215–290. doi: 10.1016/B978-0-12-407704-1.00005-1. - DOI - PMC - PubMed
-
- Cappetta D., De Angelis A., Sapio L., Prezioso L., Illiano M., Quaini F., Rossi F., Berrino L., Naviglio S., Urbanek K. Oxidative stress and cellular response to doxorubicin: a common factor in the complex milieu of anthracycline cardiotoxicity. Oxid. Med. Cell. Longev. 2017;2017:1521020. doi: 10.1155/2017/1521020. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

