Tau seeds from Alzheimer's disease brains trigger tau spread in macaques while oligomeric-Aβ mediates pathology maturation
- PMID: 38148705
- PMCID: PMC10984505
- DOI: 10.1002/alz.13604
Tau seeds from Alzheimer's disease brains trigger tau spread in macaques while oligomeric-Aβ mediates pathology maturation
Abstract
Introduction: The "prion-like" features of Alzheimer's disease (AD) tauopathy and its relationship with amyloid-β (Aβ) have never been experimentally studied in primates phylogenetically close to humans.
Methods: We injected 17 macaques in the entorhinal cortex with nanograms of seeding-competent tau aggregates purified from AD brains or control extracts from aged-matched healthy brains, with or without intracerebroventricular co-injections of oligomeric-Aβ.
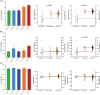
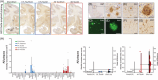
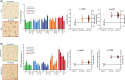
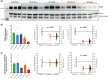
Results: Pathological tau injection increased cerebrospinal fluid (CSF) p-tau181 concentration after 18 months. Tau pathology spreads from the entorhinal cortex to the hippocampal trisynaptic loop and the cingulate cortex, resuming the experimental progression of Braak stage I to IV. Many AD-related molecular networks were impacted by tau seeds injections regardless of Aβ injections in proteomic analyses. However, we found mature neurofibrillary tangles, increased CSF total-tau concentration, and pre- and postsynaptic degeneration only in Aβ co-injected macaques.
Discussion: Oligomeric-Aβ mediates the maturation of tau pathology and its neuronal toxicity in macaques but not its initial spreading.
Highlights: This study supports the "prion-like" properties of misfolded tau extracted from AD brains. This study empirically validates the Braak staging in an anthropomorphic brain. This study highlights the role of oligomeric Aβ in driving the maturation and toxicity of tau pathology. This work establishes a novel animal model of early sporadic AD that is closer to the human pathology.
Keywords: Alzheimer's disease; beta-amyloid; non-human primate; prion-like; tau; tauopathy.
© 2024 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Erwan Bezard is a director and shareholder of Motac Neuroscience Ltd. During the past 3 years, Vincent Planche was a local unpaid investigator or sub‐investigator for clinical trials granted by Novo Nordisk, Biogen, TauRx Pharmaceuticals, Janssen, Green Valley Pharmaceuticals, and Alector. Vincent Planche served as a consultant for Motac Neuroscience Ltd, outside the submitted work. The other authors declare no conflict of interest. Author disclosures are available in the supporting information.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

