Emerging and Fastidious Uropathogens Were Detected by M-PCR with Similar Prevalence and Cell Density in Catheter and Midstream Voided Urine Indicating the Importance of These Microbes in Causing UTIs
- PMID: 38148772
- PMCID: PMC10750486
- DOI: 10.2147/IDR.S429990
Emerging and Fastidious Uropathogens Were Detected by M-PCR with Similar Prevalence and Cell Density in Catheter and Midstream Voided Urine Indicating the Importance of These Microbes in Causing UTIs
Abstract
Introduction: This study compared microbial compositions of midstream and catheter urine specimens from patients with suspected complicated urinary tract infections to determine if emerging and fastidious uropathogens are infecting the bladder or are contaminants.
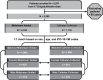
Methods: Urine was collected by in-and-out catheter (n = 1000) or midstream voiding (n = 1000) from 2000 adult patients (≥60 years of age) at 17 DispatchHealth sites across 11 states. The two groups were matched by age (mean 81 years), sex (62.1% female, 37.9% male), and ICD-10-CM codes. Microbial detection was performed with multiplex polymerase chain reaction (M-PCR) with a threshold for "positive detection" ≥ 10,000 cells/mL for bacteria or any detection for yeast. Results were divided by sex.
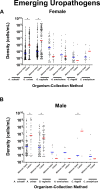
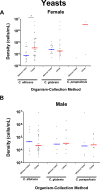
Results: In females, 28 of 30 microorganisms/groups were found by both collection methods, while in males 26 of 30 were found by both. There were significant overlaps in the detection and densities of classical uropathogens including Escherichia coli, Enterococcus faecalis, and Klebsiella pneumoniae, as well as emerging uropathogens including Actinotignum schaalii and Aerococcus urinae. In females, detection rates were slightly higher in midstream voided compared to catheter-collected (p = 0.0005) urine samples, while males showed the opposite trend (p < 0.0001). More polymicrobial infections were detected in midstream voided compared to catheter-collected samples (64.4% vs 45.7%, p < 0.0001) in females but the opposite in males (35.6% vs 47.0%, p = 0.002).
Discussion: In-and-out catheter-collected and midstream voided urine specimens shared significant similarities in microbial detections by M-PCR, with some differences found for a small subset of organisms and between sexes.
Conclusion: Non-invasive midstream voided collection of urine specimens for microbial detection and identification in cases of presumed UTI does not result in significantly more contamination compared to in-and-out catheter-collected specimens. Additionally, organisms long regarded as contaminants should be reconsidered as potential uropathogens.
Keywords: catheter; diagnostic testing; midstream voided; multiplex polymerase chain reaction; standard urine culture; urinary tract infection.
© 2023 Wang et al.
Conflict of interest statement
D.B., N.L., E.H., R.A.F., and M.M. are employees of Pathnostics, and D.W. and X.Z. are paid consultants of Pathnostics. L.A.A. reports personal fees from Pathnostics, outside the submitted work. In addition, N.L. has patents (10,160,991, 11,053,532, 17/335,767, 63/493,416, AU2018254514 B2, BR112019021943-9 B1 and NZ 759292) issued to Pathnostics; pending patents (17/178,091 17/880,227 63/503,939) to Pathnostics. D.B. reports patents (US10160991, US11053532, US17178091, US17335767, AU2018254514B2, BR1120190219439B1, NZ759292) issued to PATHNOSTICS; pending patents (US17830227, US18351385, US18351286, US63493416, US63503393, US63514785, PCTUS2216816, PCTUS2277477, EP3612638, JP2022042545, CA3175879, CA3176586, CA3061015, HK620200143373, CN2018800399569, IL294577) to PATHNOSTICS. The authors report no other conflicts of interest in this work.
Figures


References
LinkOut - more resources
Full Text Sources

