Current status of Er:YAG laser in periodontal surgery
- PMID: 38148873
- PMCID: PMC10750110
- DOI: 10.1016/j.jdsr.2023.11.002
Current status of Er:YAG laser in periodontal surgery
Abstract
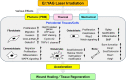
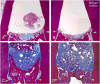
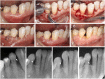
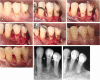
Lasers have numerous advantageous tissue interactions such as ablation or vaporization, hemostasis, bacterial killing, as well as biological effects, which induce various beneficial therapeutic effects and biological responses in the tissues. Thus, lasers are considered an effective and suitable device for treating a variety of inflammatory and infectious conditions of periodontal disease. Among various laser systems, the Er:YAG laser, which can be effectively and safely used in both soft and hard tissues with minimal thermal side effects, has been attracting much attention in periodontal therapy. This laser can effectively and precisely debride the diseased root surface including calculus removal, ablate diseased connective tissues within the bone defects, and stimulate the irradiated surrounding periodontal tissues during surgery, resulting in favorable wound healing as well as regeneration of periodontal tissues. The safe and effective performance of Er:YAG laser-assisted periodontal surgery has been reported with comparable and occasionally superior clinical outcomes compared to conventional surgery. This article explains the characteristics of the Er:YAG laser and introduces its applications in periodontal surgery including conventional flap surgery, regenerative surgery, and flapless surgery, based on scientific evidence from currently available basic and clinical studies as well as cases reports.
Keywords: Lasers; Periodontal debridement; Periodontal surgery; Periodontitis; Regeneration; Wound healing.
© 2023 The Authors.
Conflict of interest statement
none.
Figures

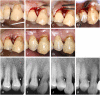

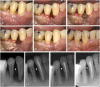
Similar articles
-
Periodontal and peri-implant wound healing following laser therapy.Periodontol 2000. 2015 Jun;68(1):217-69. doi: 10.1111/prd.12080. Periodontol 2000. 2015. PMID: 25867988
-
Clinical application of erbium:YAG laser in periodontology.J Int Acad Periodontol. 2008 Jan;10(1):22-30. J Int Acad Periodontol. 2008. PMID: 18333597 Review.
-
Residual periodontal pocket treatment with Er:YAG laser-assisted comprehensive periodontal pocket therapy: a retrospective study.Clin Oral Investig. 2022 Jan;26(1):761-771. doi: 10.1007/s00784-021-04054-9. Epub 2021 Jul 19. Clin Oral Investig. 2022. PMID: 34278521
-
Periodontal tissue healing following flap surgery using an Er:YAG laser in dogs.Lasers Surg Med. 2006 Apr;38(4):314-24. doi: 10.1002/lsm.20299. Lasers Surg Med. 2006. PMID: 16568444
-
Calculus Removal and Root Surface Roughness When Using the Er:YAG or Er,Cr:YSGG Laser Compared with Conventional Instrumentation Method: A Literature Review.Photobiomodul Photomed Laser Surg. 2019 Apr;37(4):197-226. doi: 10.1089/photob.2018.4465. Photobiomodul Photomed Laser Surg. 2019. PMID: 31050960 Review.
Cited by
-
The impact of personalized postoperative care plan on the compliance and long-term efficacy of minimally invasive periodontal surgery treatment recommendations.Eur J Med Res. 2025 May 27;30(1):420. doi: 10.1186/s40001-025-02701-5. Eur J Med Res. 2025. PMID: 40420246 Free PMC article.
-
Comparative evaluation of the effect of impregnated retraction cord versus laser on gingival attachment level and pain perception following retraction for subgingival margins - A prospective, split-mouth, controlled, clinical study.J Indian Prosthodont Soc. 2024 Apr 1;24(2):136-143. doi: 10.4103/jips.jips_437_23. Epub 2024 Apr 23. J Indian Prosthodont Soc. 2024. PMID: 38650338 Free PMC article.
-
Er:YAG laser biofilm removal from zero-gap periodontal/peri-implant model system mimicking clinical attachment loss.J Biomed Opt. 2025 Feb;30(2):025002. doi: 10.1117/1.JBO.30.2.025002. Epub 2025 Feb 25. J Biomed Opt. 2025. PMID: 40008293 Free PMC article.
-
Passive Eruption of a Soft-Tissue-Impacted Maxillary Canine Following Diode Laser Exposure: A Case Report.Cureus. 2025 Jun 7;17(6):e85526. doi: 10.7759/cureus.85526. eCollection 2025 Jun. Cureus. 2025. PMID: 40625479 Free PMC article.
-
The effect of surface roughness on the Er:YAG laser-induced photoacoustic removal of bacteria in zero-gap periodontal/peri-implant pocket model.Ultrason Sonochem. 2025 Jul 7;120:107458. doi: 10.1016/j.ultsonch.2025.107458. Online ahead of print. Ultrason Sonochem. 2025. PMID: 40645117 Free PMC article.
References
-
- Feres M., Figueiredo L.C., Soares G.M., Faveri M. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000. 2015;67(1):131–186. - PubMed
-
- Graziani F., Karapetsa D., Alonso B., Herrera D. Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontol 2000. 2017;75(1):152–188. - PubMed
-
- Pick R.M., Pecaro B.C., Silberman C.J. The laser gingivectomy. The use of the CO2 laser for the removal of phenytoin hyperplasia. J Periodo. 1985;56(8):492–496. - PubMed
-
- Pick R.M., Pecaro B.C. Use of the CO2 laser in soft tissue dental surgery. Lasers Surg Med. 1987;7(2):207–213. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

