Drug transport by red blood cells
- PMID: 38148901
- PMCID: PMC10750411
- DOI: 10.3389/fphys.2023.1308632
Drug transport by red blood cells
Erratum in
-
Corrigendum: Drug transport by red blood cells.Front Physiol. 2024 Jul 30;15:1454770. doi: 10.3389/fphys.2024.1454770. eCollection 2024. Front Physiol. 2024. PMID: 39139478 Free PMC article.
Abstract
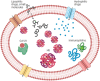
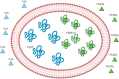
This review focuses on the role of human red blood cells (RBCs) as drug carriers. First, a general introduction about RBC physiology is provided, followed by the presentation of several cases in which RBCs act as natural carriers of drugs. This is due to the presence of several binding sites within the same RBCs and is regulated by the diffusion of selected compounds through the RBC membrane and by the presence of influx and efflux transporters. The balance between the influx/efflux and the affinity for these binding sites will finally affect drug partitioning. Thereafter, a brief mention of the pharmacokinetic profile of drugs with such a partitioning is given. Finally, some examples in which these natural features of human RBCs can be further exploited to engineer RBCs by the encapsulation of drugs, metabolites, or target proteins are reported. For instance, metabolic pathways can be powered by increasing key metabolites (i.e., 2,3-bisphosphoglycerate) that affect oxygen release potentially useful in transfusion medicine. On the other hand, the RBC pre-loading of recombinant immunophilins permits increasing the binding and transport of immunosuppressive drugs. In conclusion, RBCs are natural carriers for different kinds of metabolites and several drugs. However, they can be opportunely further modified to optimize and improve their ability to perform as drug vehicles.
Keywords: blood distribution; blood-to-plasma ratio; drug transport; pharmacokinetic; red blood cells.
Copyright © 2023 Biagiotti, Pirla and Magnani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Anastasiadi A. T., Stamoulis K., Papageorgiou E. G., Lelli V., Rinalducci S., Papassideri I. S., et al. (2023). The time-course linkage between hemolysis, redox, and metabolic parameters during red blood cell storage with or without uric acid and ascorbic acid supplementation. Front. Aging 4, 1161565. 10.3389/fragi.2023.1161565 - DOI - PMC - PubMed
-
- Ataullakhanov F. I., Batasheva T. V., Vitvitskii V. M. (1994). Effect of temperature, daunorubicin concentration and suspension hematocrit on daunorubicin binding by human erythrocytes. Antibiot. Khimioter 39 (9-10), 26–29. - PubMed
-
- Ataullakhanov F. I., Isaev V. G., Kohno A. V., Kulikova E. V., Parovichnikova E. N., Savchenko V. G., et al. (1997). Pharmacokinetics of doxorubicin in patients with lymphoproliferative disorders after infusion of doxorubicin-loaded erythrocytes. Erythrocytes as Drug Carriers Med., 137–142. 10.1007/978-1-4899-0044-9_18 - DOI
Publication types
LinkOut - more resources
Full Text Sources

