Differential Myosin 5a splice variants in innervation of pelvic organs
- PMID: 38148903
- PMCID: PMC10749955
- DOI: 10.3389/fphys.2023.1304537
Differential Myosin 5a splice variants in innervation of pelvic organs
Abstract
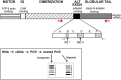
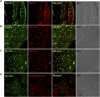
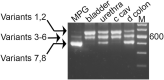
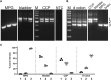
Introduction: Myosin proteins interact with filamentous actin and translate the chemical energy generated by ATP hydrolysis into a wide variety of mechanical functions in all cell types. The classic function of conventional myosins is mediation of muscle contraction, but myosins also participate in processes as diverse as exocytosis/endocytosis, membrane remodeling, and cytokinesis. Myosin 5a (Myo5a) is an unconventional motor protein well-suited to the processive transport of diverse molecular cargo within cells and interactions with multiprotein membrane complexes that facilitate exocytosis. Myo5a includes a region consisting of six small alternative exons which can undergo differential splicing. Neurons and skin melanocytes express characteristic splice variants of Myo5a, which are specialized for transport processes unique to those cell types. But less is known about the expression of Myo5a splice variants in other tissues, their cargos and interactive partners, and their regulation. Methods: In visceral organs, neurotransmission-induced contraction or relaxation of smooth muscle is mediated by Myo5a. Axons within urogenital organs and distal colon of rodents arise from cell bodies located in the major pelvic ganglion (MPG). However, in contrast to urogenital organs, the distal colon also contains soma of the enteric nervous system. Therefore, the rodent pelvic organs provide an opportunity to compare the expression of Myo5a splice variants, not only in different tissues innervated by the pelvic nerves, but also in different subcellular compartments of those nerves. This study examines the expression and distribution of Myo5a splice variants in the MPG, compared to the bladder, corpus cavernosum of the penis (CCP) and distal colon using immunohistochemistry and mRNA analyses. Results/discussion: We report detection of characteristic Myo5a variants in these tissues, with bladder and CCP displaying a similar variant pattern but one which differed from that of distal colon. In all three organs, Myo5a variants were distinct compared to the MPG, implying segregation of one variant within nerve soma and its exclusion from axons. The expression of distinct Myo5a variant arrays is likely to be adaptive, and to underlie specific functions fulfilled by Myo5a in those particular locations.
Keywords: myosin motor; neurotransmission; pelvic organ; peripheral nerve; protein splice variants.
Copyright © 2023 Carew, Cristofaro, Goyal and Sullivan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Expression of Myosin 5a splice variants in murine stomach.Neurogastroenterol Motil. 2021 Oct;33(10):e14162. doi: 10.1111/nmo.14162. Epub 2021 May 3. Neurogastroenterol Motil. 2021. PMID: 33939222
-
Myosin 5a in the Urinary Bladder: Localization, Splice Variant Expression, and Functional Role in Neurotransmission.Front Physiol. 2022 Jul 1;13:890102. doi: 10.3389/fphys.2022.890102. eCollection 2022. Front Physiol. 2022. PMID: 35845995 Free PMC article.
-
Myosin Va associates with mRNA in ribonucleoprotein particles present in myelinated peripheral axons and in the central nervous system.Dev Neurobiol. 2014 Mar;74(3):382-96. doi: 10.1002/dneu.22155. Epub 2014 Jan 27. Dev Neurobiol. 2014. PMID: 24272908
-
Cargo recognition and cargo-mediated regulation of unconventional myosins.Acc Chem Res. 2014 Oct 21;47(10):3061-70. doi: 10.1021/ar500216z. Epub 2014 Sep 17. Acc Chem Res. 2014. PMID: 25230296 Review.
-
Organization of lumbar spinal outflow to distal colon and pelvic organs.Physiol Rev. 1987 Oct;67(4):1332-404. doi: 10.1152/physrev.1987.67.4.1332. Physiol Rev. 1987. PMID: 2891149 Review.
Cited by
-
Applying the algorithm for Proven and young in GWAS Reveals high polygenicity for key traits in Nellore cattle.Front Genet. 2025 Apr 30;16:1549284. doi: 10.3389/fgene.2025.1549284. eCollection 2025. Front Genet. 2025. PMID: 40370699 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

