Method to Regulate Monocyte Function by Silencing HIF-1α mRNA in a Model of Retinal Neovascularization
- PMID: 38148985
- PMCID: PMC10749564
- DOI: 10.1021/acsanm.3c04300
Method to Regulate Monocyte Function by Silencing HIF-1α mRNA in a Model of Retinal Neovascularization
Abstract
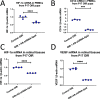
Circulating monocytes migrate into the retina in response to inflammation and neovascularization. Furthermore, under inflammatory conditions such as diabetes, healthy monocytes become activated in the circulation. However, the contribution of activated monocytes to neovascularization is largely unknown. HIF-1α has been shown to contribute to the pathogenesis of neovascularization. We describe here the synthesis of a hybrid nanomaterial for targeted delivery and gene silencing in activated monocytes that are associated with pathological neovascularization. To test the gene silencing ability of AS-shRNA-lipids in vitro, we used the probe to inhibit HIF-1α mRNA induced in mouse monocytes by exposing them to hypoxia. In addition, we tested AS-shRNA-lipids for inhibition of neovascularization in vivo using the mouse model of oxygen-induced retinopathy (OIR). Significant reduction of neovascularization was achieved in mouse OIR by targeting activated monocytes using intraperitoneal injections of AS-shRNA-lipids. Expression of HIF-1α and CD14 mRNA were both inhibited in circulating cells, suggesting normalization of the activated monocytes in P17 OIR animals treated with AS-shRNA-lipids. We hypothesized that inhibition of HIF-1α mRNA in activated monocytes may have a direct impact on VEGF expression in the retinal tissues in vivo. We observed that VEGF mRNA expression was inhibited in P17 retinal tissues after systemic treatment with HIF-1α-targeted AS-shRNA-lipids. These findings may provide a framework for a strategy to inhibit retinal neovascularization by targeting circulating activated monocytes.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Campbell M.; Humphries P.. THE BLOOD-RETINA BARRIER Tight Junctions and Barrier Modulation. In Advances in Experimental Medicine and Biology; Springer, 2013; Vol. 763, pp 70–84. - PubMed
-
- Davies M.; Eubanks J.; Powers M. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol. Vision 2006, 12 (53–54), 467–477. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
