Development and validation of a rabbit model of Pseudomonas aeruginosa non-ventilated pneumonia for preclinical drug development
- PMID: 38149013
- PMCID: PMC10750358
- DOI: 10.3389/fcimb.2023.1297281
Development and validation of a rabbit model of Pseudomonas aeruginosa non-ventilated pneumonia for preclinical drug development
Abstract
Background: New drugs targeting antimicrobial resistant pathogens, including Pseudomonas aeruginosa, have been challenging to evaluate in clinical trials, particularly for the non-ventilated hospital-acquired pneumonia and ventilator-associated pneumonia indications. Development of new antibacterial drugs is facilitated by preclinical animal models that could predict clinical efficacy in patients with these infections.
Methods: We report here an FDA-funded study to develop a rabbit model of non-ventilated pneumonia with Pseudomonas aeruginosa by determining the extent to which the natural history of animal disease reproduced human pathophysiology and conducting validation studies to evaluate whether humanized dosing regimens of two antibiotics, meropenem and tobramycin, can halt or reverse disease progression.
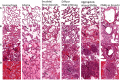
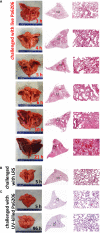
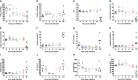
Results: In a rabbit model of non-ventilated pneumonia, endobronchial challenge with live P. aeruginosa strain 6206, but not with UV-killed Pa6206, caused acute respiratory distress syndrome, as evidenced by acute lung inflammation, pulmonary edema, hemorrhage, severe hypoxemia, hyperlactatemia, neutropenia, thrombocytopenia, and hypoglycemia, which preceded respiratory failure and death. Pa6206 increased >100-fold in the lungs and then disseminated from there to infect distal organs, including spleen and kidneys. At 5 h post-infection, 67% of Pa6206-challenged rabbits had PaO2 <60 mmHg, corresponding to a clinical cut-off when oxygen therapy would be required. When administered at 5 h post-infection, humanized dosing regimens of tobramycin and meropenem reduced mortality to 17-33%, compared to 100% for saline-treated rabbits (P<0.001 by log-rank tests). For meropenem which exhibits time-dependent bactericidal activity, rabbits treated with a humanized meropenem dosing regimen of 80 mg/kg q2h for 24 h achieved 100% T>MIC, resulting in 75% microbiological clearance rate of Pa6206 from the lungs. For tobramycin which exhibits concentration-dependent killing, rabbits treated with a humanized tobramycin dosing regimen of 8 mg/kg q8h for 24 h achieved Cmax/MIC of 9.8 ± 1.4 at 60 min post-dose, resulting in 50% lung microbiological clearance rate. In contrast, rabbits treated with a single tobramycin dose of 2.5 mg/kg had Cmax/MIC of 7.8 ± 0.8 and 8% (1/12) microbiological clearance rate, indicating that this rabbit model can detect dose-response effects.
Conclusion: The rabbit model may be used to help predict clinical efficacy of new antibacterial drugs for the treatment of non-ventilated P. aeruginosa pneumonia.
Keywords: Pseudomonas aeruginosa; meropenem; non-ventilated pneumonia model; preclinical efficacy model validation; rabbit model; tobramycin.
Copyright © 2023 Gras, Vu, Nguyen, Tran, Mao, Tran, Mai, Dong, Jung, Iorio, Povoa, Pinheiro, Aguiar-Alves, Weiss, Zheng, Cheng, Stover, Sellman, DiGiandomenico, Gibault, Valour and Diep.
Conflict of interest statement
Authors BZ, LC, CS, AD and BS are or were employed by AstraZeneca. Author BD previously received funding from AstraZeneca, Pfizer, Merck, Arsanis, ContraFect, and Integrated Biotherapeutics for preclinical studies using earlier iterations of the rabbit model of non-ventilated pneumonia. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
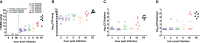


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

