Pharmacological activation of constitutive androstane receptor induces female-specific modulation of hepatic metabolism
- PMID: 38149074
- PMCID: PMC10749885
- DOI: 10.1016/j.jhepr.2023.100930
Pharmacological activation of constitutive androstane receptor induces female-specific modulation of hepatic metabolism
Abstract
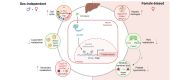
Background & aims: The constitutive androstane receptor (CAR) is a nuclear receptor that binds diverse xenobiotics and whose activation leads to the modulation of the expression of target genes involved in xenobiotic detoxification and energy metabolism. Although CAR hepatic activity is considered to be higher in women than in men, its sex-dependent response to an acute pharmacological activation has seldom been investigated.
Methods: The hepatic transcriptome, plasma markers, and hepatic metabolome, were analysed in Car+/+ and Car-/- male and female mice treated either with the CAR-specific agonist 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) or with vehicle.
Results: Although 90% of TCPOBOP-sensitive genes were modulated in a sex-independent manner, the remaining 10% showed almost exclusive female liver specificity. These female-specific CAR-sensitive genes were mainly involved in xenobiotic metabolism, inflammation, and extracellular matrix organisation. CAR activation also induced higher hepatic oxidative stress and hepatocyte cytolysis in females than in males. Hepatic expression of flavin monooxygenase 3 (Fmo3) was almost abolished and was associated with a decrease in hepatic trimethylamine-N-oxide (TMAO) concentration in TCPOBOP-treated females. In line with a potential role in the control of TMAO homeostasis, CAR activation decreased platelet hyper-responsiveness in female mice supplemented with dietary choline.
Conclusions: More than 10% of CAR-sensitive genes are sex-specific and influence hepatic and systemic responses such as platelet aggregation. CAR activation may be an important mechanism of sexually-dimorphic drug-induced liver injury.
Impact and implications: CAR is activated by many drugs and pollutants. Its pharmacological activation had a stronger impact on hepatic gene expression and metabolism in females than in males, and had a specific impact on liver toxicity and trimethylamine metabolism. Sexual dimorphism should be considered when testing and/or prescribing xenobiotics known to activate CAR.
Keywords: Hepatic xenobiotic metabolism; Lipoprotein metabolism; Platelet aggregation; Sexual dimorphism; Trimethylamine-N-oxide.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures





References
-
- Wei P., Zhang J., Egan-Ha M., Moore D.D. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. - PubMed
-
- Moore L.B., Parks D.J., Jones S.A., et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

