Clinical development of antivirals against SARS-CoV-2 and its variants
- PMID: 38149085
- PMCID: PMC10750039
- DOI: 10.1016/j.crmicr.2023.100208
Clinical development of antivirals against SARS-CoV-2 and its variants
Abstract
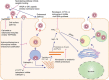
The unceasing global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) calls for the development of novel therapeutics. Although many newly developed antivirals and repurposed antivirals have been applied to the treatment of coronavirus disease 2019 (COVID-19), antivirals showing satisfactory clinical efficacy are few in number. In addition, the loss of sensitivity to variants of concern (VOCs) and lack of oral bioavailability have also limited the clinical application of some antivirals. These facts remind us to develop more potent and broad-spectrum antivirals with better pharmacokinetic/pharmacodynamic properties to fight against infections from SARS-CoV-2, its variants, and other human coronaviruses (HCoVs). In this review, we summarize the latest advancements in the clinical development of antivirals against infections by SARS-CoV-2 and its variants.
Keywords: Antivirals; COVID-19; Host factor; SARS-CoV-2; Variants; Viral factor.
© 2023 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Abbaspour Kasgari H., Moradi S., Shabani A.M., Babamahmoodi F., Davoudi Badabi A.R., Davoudi L., Alikhani A., Hedayatizadeh Omran A., Saeedi M., Merat S., Wentzel H., Garratt A., Levi J., Simmons B., Hill A., Tirgar Fakheri H. Evaluation of the efficacy of sofosbuvir plus daclatasvir in combination with ribavirin for hospitalized COVID-19 patients with moderate disease compared with standard care: a single-centre, randomized controlled trial. J. Antimicrob. Chemother. 2020;75:3373–3378. - PMC - PubMed
-
- Ader F., Bouscambert-Duchamp M., Hites M., Peiffer-Smadja N., Poissy J., Belhadi D., Diallo A., Le M.P., Peytavin G., Staub T., Greil R., Guedj J., Paiva J.A., Costagliola D., Yazdanpanah Y., Burdet C., Mentre F., DisCoVeRy Study G Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022;22:209–221. - PMC - PubMed
-
- Ahmed S., Karim M.M., Ross A.G., Hossain M.S., Clemens J.D., Sumiya M.K., Phru C.S., Rahman M., Zaman K., Somani J., Yasmin R., Hasnat M.A., Kabir A., Aziz A.B., Khan W.A. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2021;103:214–216. - PMC - PubMed
-
- Arabi Y.M., Asiri A.Y., Assiri A.M., Balkhy H.H., Al Bshabshe A., Al Jeraisy M., Mandourah Y., Azzam M.H.A., Bin Eshaq A.M., Al Johani S., Al Harbi S., Jokhdar H.A.A., Deeb A.M., Memish Z.A., Jose J., Ghazal S., Al Faraj S., Al Mekhlafi G.A., Sherbeeni N.M., Elzein F.E., Al-Hameed F., Al Saedi A., Alharbi N.K., Fowler R.A., Hayden F.G., Al-Dawood A., Abdelzaher M., Bajhmom W., AlMutairi B.M., Hussein M.A., Alothman A. Interferon beta-1b and lopinavir-ritonavir for middle east respiratory syndrome. N. Engl. J. Med. 2020;383:1645–1656. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

