Chronological age enhances aging phenomena and protein nitration in oocyte
- PMID: 38149097
- PMCID: PMC10749940
- DOI: 10.3389/fendo.2023.1251102
Chronological age enhances aging phenomena and protein nitration in oocyte
Abstract
Background: The average age of childbearing has increased over the years contributing to infertility, miscarriages, and chromosomal abnormalities largely invoked by an age-related decline in oocyte quality. In this study, we investigate the role of nitric oxide (NO) insufficiency and protein nitration in oocyte chronological aging.
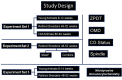
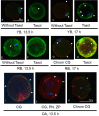
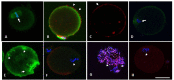
Methods: Mouse oocytes were retrieved from young breeders (YB, 8-14 weeks [w]), retired breeders (RB, 48-52w) and old animals (OA, 80-84w) at 13.5 and 17 hours after ovulation trigger. They were assessed for zona pellucida dissolution time (ZPDT); ooplasmic microtubule dynamics (OMD); cortical granule (CG) status and spindle morphology (SM), as markers of oocyte quality. Sibling oocytes from RB were exposed to NO supplementation and assessed for aging phenomena (AP). All oocyte cumulus complexes were subjected to fluorescence nitrotyrosine (NT) immunocytochemistry and confocal microscopy to assess morphology and protein nitration.
Results: At 13.5 h from hCG trigger, oocytes from RB compared to YB had significantly increased ZPDT (37.8 ± 11.9 vs 22.1 ± 4.1 seconds [s]), OMD (46.9 vs 0%), CG loss (39.4 vs 0%), and decreased normal SM (30.3 vs 81.3%), indicating premature AP that worsened among oocytes from RB at 17 hours post-hCG trigger. When exposed to SNAP, RB AP significantly decreased (ZPDT: 35.1 ± 5.5 vs 46.3 ± 8.9s, OMD: 13.3 vs 75.0% and CG loss: 50.0 vs 93.3%) and SM improved (80.0 vs 14.3%). The incidence of NT positivity was significantly higher in cumulus cells (13.5 h, 46.7 ± 4.5 vs 3.4 ± 0.7%; 17 h, 82.2 ± 2.9 vs 23.3 ± 3.6%) and oocytes (13.5 h, 57.1 vs 0%; 17 h, 100.0 vs 55.5%) from RB compared to YB. Oocytes retrieved decreased with advancing age (29.8 ± 4.1 per animal in the YB group compared to 10.2 ± 2.1 in RB and 4.0 ± 1.6 in OA). Oocytes from OA displayed increased ZPDT, major CG loss, increased OMD and spindle abnormalities, as well as pronuclear formation, confirming spontaneous meiosis to interphase transition.
Conclusions: Oocytes undergo zona pellucida hardening, altered spindle and ooplasmic microtubules, and premature cortical granule release, indicative of spontaneous meiosis-interphase transition, as a function of chronological aging. These changes are also associated with NO insufficiency and protein nitration and may be alleviated through supplementation with an NO-donor.
Keywords: advanced reproductive age; nitric oxide; oocyte aging; oocyte quality; oocyte temporal window.
Copyright © 2023 Goud, Goud, Camp, Bai, Gonik, Diamond and Abu-Soud.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Why American Women Everywhere Are Delaying Motherhood (2021). Available at: https://www.nytimes.com/2021/06/16/us/declining-birthrate-motherhood.htm....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

