A semi-quantitative upconversion nanoparticle-based immunochromatographic assay for SARS-CoV-2 antigen detection
- PMID: 38149276
- PMCID: PMC10750388
- DOI: 10.3389/fmicb.2023.1289682
A semi-quantitative upconversion nanoparticle-based immunochromatographic assay for SARS-CoV-2 antigen detection
Abstract
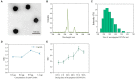
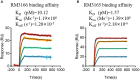
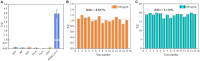
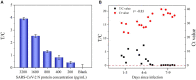
The unprecedented public health and economic impact of the coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been met with an equally unprecedented scientific response. Sensitive point-of-care methods to detect SARS-CoV-2 antigens in clinical specimens are urgently required for the rapid screening of individuals with viral infection. Here, we developed an upconversion nanoparticle-based lateral flow immunochromatographic assay (UCNP-LFIA) for the high-sensitivity detection of SARS-CoV-2 nucleocapsid (N) protein. A pair of rabbit SARS-CoV-2 N-specific monoclonal antibodies was conjugated to UCNPs, and the prepared UCNPs were then deposited into the LFIA test strips for detecting and capturing the N protein. Under the test conditions, the limit of detection (LOD) of UCNP-LFIA for the N protein was 3.59 pg/mL, with a linear range of 0.01-100 ng/mL. Compared with that of the current colloidal gold-based LFIA strips, the LOD of the UCNP-LFIA-based method was increased by 100-fold. The antigen recovery rate of the developed method in the simulated pharyngeal swab samples ranged from 91.1 to 117.3%. Furthermore, compared with the reverse transcription-polymerase chain reaction, the developed UCNP-LFIA method showed a sensitivity of 94.73% for 19 patients with COVID-19. Thus, the newly established platform could serve as a promising and convenient fluorescent immunological sensing approach for the efficient screening and diagnosis of COVID-19.
Keywords: SARS-CoV-2; antigen detection; fluorescence immunochromatography assay; semi-quantitative; upconversion nanoparticles.
Copyright © 2023 Ding, Zhang, Wang, Li, Li, Liu, Yu, Tao, Cheng, Xie and Chen.
Conflict of interest statement
JL, FY, and YT were employed by Polariton Life Technologies Ltd., Soochow. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Gold Nanoparticle-Based Colorimetric and Fluorescent Dual-Mode Lateral Flow Immunoassay for SARS-CoV-2 Detection.J Funct Biomater. 2024 Feb 27;15(3):58. doi: 10.3390/jfb15030058. J Funct Biomater. 2024. PMID: 38535251 Free PMC article.
-
Ultrasensitive and Simultaneous Detection of Two Specific SARS-CoV-2 Antigens in Human Specimens Using Direct/Enrichment Dual-Mode Fluorescence Lateral Flow Immunoassay.ACS Appl Mater Interfaces. 2021 Sep 1;13(34):40342-40353. doi: 10.1021/acsami.1c11461. Epub 2021 Aug 19. ACS Appl Mater Interfaces. 2021. PMID: 34412466
-
Au@Pt@Pd nanozymes based lateral flow immunoassay for quantitative detection of SARS-CoV-2 nucleocapsid protein in nasal swab samples.Mikrochim Acta. 2024 Nov 7;191(12):730. doi: 10.1007/s00604-024-06819-x. Mikrochim Acta. 2024. PMID: 39508966
-
Development and Efficacy of Lateral Flow Point-of-Care Testing Devices for Rapid and Mass COVID-19 Diagnosis by the Detections of SARS-CoV-2 Antigen and Anti-SARS-CoV-2 Antibodies.Diagnostics (Basel). 2021 Sep 24;11(10):1760. doi: 10.3390/diagnostics11101760. Diagnostics (Basel). 2021. PMID: 34679458 Free PMC article. Review.
-
Immunochromatographic enhancement strategy for SARS-CoV-2 detection based on nanotechnology.Nanoscale. 2023 Sep 29;15(37):15092-15107. doi: 10.1039/d3nr02396f. Nanoscale. 2023. PMID: 37676509 Review.
Cited by
-
Development of Detection Antibody Targeting the Linear Epitope in SARS-CoV-2 Nucleocapsid Protein with Ultra-High Sensitivity.Int J Mol Sci. 2024 Apr 18;25(8):4436. doi: 10.3390/ijms25084436. Int J Mol Sci. 2024. PMID: 38674021 Free PMC article.
-
Upconversion Nanoparticle-Based Dot-Blot Immunoassay for Quantitative Biomarker Detection.Anal Chem. 2024 Jun 25;96(25):10237-10245. doi: 10.1021/acs.analchem.4c00837. Epub 2024 Jun 13. Anal Chem. 2024. PMID: 38870418 Free PMC article.
-
Designing and expression of novel recombinant fusion protein for efficient antigen screening of SARS-CoV-2.AMB Express. 2024 Jul 11;14(1):80. doi: 10.1186/s13568-024-01719-y. AMB Express. 2024. PMID: 38990364 Free PMC article.
References
-
- Chen C., Jiao C., Xiaochen W., Qian C., Yunjuan Z., Hongzhong C., et al. . (2022). A novel dual-flux immunochromatographic test strip based on luminescence resonance energy transfer for simultaneous detection of ochratoxin a and deoxynivalenol. Mikrochim. Acta 189:466. doi: 10.1007/s00604-022-05561-6, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

