Behavioral observations, heart rate and heart rate variability in horses following oral administration of a cannabidiol containing paste in three escalating doses (part 1/2)
- PMID: 38149295
- PMCID: PMC10750369
- DOI: 10.3389/fvets.2023.1305868
Behavioral observations, heart rate and heart rate variability in horses following oral administration of a cannabidiol containing paste in three escalating doses (part 1/2)
Abstract
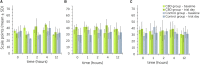
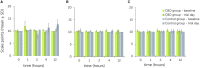
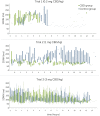
Cannabidiol (CBD) products have been proposed to exert stress- and anxiety-relieving effects in animals. Despite the increasing popularity of CBD for veterinary use, the available research detailing the effects of CBD in horses is limited. The aim of this study (part 1 of 2) was to analyze stress parameters via behavioral observations and heart rate monitoring in healthy horses following single oral administration of a CBD containing paste in different doses. Study products were two pastes for oral administration, one containing CBD and one containing no active ingredient. Pastes were applied as single administrations in consecutive trials with escalating dosages (doses: 0.2, 1.0, 3.0 mg CBD/kg) to a treatment (trial 1: n = 3, trial 2: n = 3, trial 3: n = 5 horses) and a control group (trial 1: n = 3, trial 2: n = 3, trial 3: n = 6 horses) with minimum wash-out periods of seven days in between. Behavioral parameters were evaluated using video recordings to score the levels of sedation including the horses' reactions to acoustic and visual stimuli. Facial expression was assessed using photographs. Evaluation was based on the previously described facial sedation scale for horses (FaceSed) and the Horse Grimace Scale. For baseline values, identical observations were recorded on the day before each paste administration. Both paste administration and behavioral evaluation were performed double blinded. Cardiac beat-to-beat (R-R) intervals were continuously recorded throughout the trial and assessed using heart rate and heart rate variability parameters. Statistical analysis included comparison between treatment and control group over escalating doses and time points using linear mixed models. The CBD paste was well tolerated, and no side effects were observed. Analysis of sedation scores and facial expressions did not indicate significant differences between treatment and control group over the escalating doses. The heart rate was neither reduced, nor were significant changes in heart rate variability observed compared to the control group. Main limitation of this study is the small sample size. Further research is required to determine adequate doses and indications for the use of CBD products in horses.
Keywords: CBD; FaceSed; Horse Grimace Scale; behavior; equine; sedation score.
Copyright © 2023 Eichler, Ehrle, Jensen, Baudisch, Petersen, Bäumer, Lischer and Wiegard.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. This study received funding from Herosan healthcare GmbH. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. All authors declare to have full control over the data and no other competing interests.
Figures



Similar articles
-
Behavioral observations, heart rate and cortisol monitoring in horses following multiple oral administrations of a cannabidiol containing paste (part 2/2).Front Vet Sci. 2024 Jan 3;10:1305873. doi: 10.3389/fvets.2023.1305873. eCollection 2023. Front Vet Sci. 2024. PMID: 38234983 Free PMC article.
-
Pharmacokinetic modelling of orally administered cannabidiol and implications for medication control in horses.Front Vet Sci. 2023 Aug 9;10:1234551. doi: 10.3389/fvets.2023.1234551. eCollection 2023. Front Vet Sci. 2023. PMID: 37621871 Free PMC article.
-
Effects of a Supplement Containing Cannabidiol (CBD) on Sedation and Ataxia Scores and Health.J Equine Vet Sci. 2022 Oct;117:104085. doi: 10.1016/j.jevs.2022.104085. Epub 2022 Jul 23. J Equine Vet Sci. 2022. PMID: 35882292
-
Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy.Drugs. 2019 Sep;79(13):1435-1454. doi: 10.1007/s40265-019-01171-4. Drugs. 2019. PMID: 31372958 Review.
-
Use of cannabidiol in anxiety and anxiety-related disorders.J Am Pharm Assoc (2003). 2020 Jan-Feb;60(1):253-261. doi: 10.1016/j.japh.2019.11.008. Epub 2019 Dec 19. J Am Pharm Assoc (2003). 2020. PMID: 31866386
Cited by
-
Behavioral observations, heart rate and cortisol monitoring in horses following multiple oral administrations of a cannabidiol containing paste (part 2/2).Front Vet Sci. 2024 Jan 3;10:1305873. doi: 10.3389/fvets.2023.1305873. eCollection 2023. Front Vet Sci. 2024. PMID: 38234983 Free PMC article.
-
Improved quality of life and pain relief in mature horses with osteoarthritis after oral transmucosal cannabidiol oil administration as part of an analgesic regimen.Front Vet Sci. 2024 Feb 6;11:1341396. doi: 10.3389/fvets.2024.1341396. eCollection 2024. Front Vet Sci. 2024. PMID: 38379920 Free PMC article.
-
Endocannabinoid system and phytocannabinoids in the main species of veterinary interest: a comparative review.Vet Res Commun. 2024 Oct;48(5):2915-2941. doi: 10.1007/s11259-024-10509-7. Epub 2024 Aug 20. Vet Res Commun. 2024. PMID: 39162768 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

