Cordyceps cicadae polysaccharides attenuate diabetic nephropathy via the miR-30a-3p/TRIM16 axis
- PMID: 38149724
- PMCID: PMC10906025
- DOI: 10.1111/jdi.14116
Cordyceps cicadae polysaccharides attenuate diabetic nephropathy via the miR-30a-3p/TRIM16 axis
Abstract
Objective: The molecular mechanism of the protective effect of Cordyceps cicadae polysaccharides (CCPs) on renal tubulointerstitial fibrosis in diabetic nephropathy (DN) is still unclear. This study aims to further understand the molecular mechanisms behind the therapeutic benefits of CCP on diabetic nephropathy.
Methods: Mice were randomly assigned into six groups (n = 8). Cordyceps cicadae polysaccharide dissolved in 5% dimethyl sulfoxide was administered by gavage for 12 consecutive weeks. The CCP doses were divided into low, medium, and high, 75, 150, and 300 mg/kg/day, respectively. The efficacy of CCP was determined by assessing the renal function and histological alterations in diabetic db/db mice. The degree of glomerular mesangial dilatation and sclerosis was evaluated using semiquantitative markers. Cell viability, apoptosis, epithelial-mesenchymal transition (EMT), inflammation, oxidative stress, and mitochondrial reactive oxygen species (ROS) in high glucose (HG)-cultured MPC5 podocytes were determined. The interaction of miR-30a-3p and tripartite motif-containing protein 16 (TRIM16) was examined by luciferase reporter assay. Western blotting, reverse transcription-polymerase chain reaction, and immunofluorescence were used to analyze gene and protein expressions.
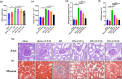
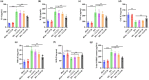
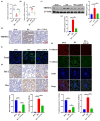
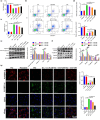
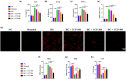
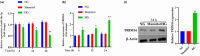
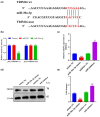
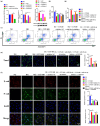
Results: The in vivo findings illustrated that CCP may protect mice with type 2 diabetes from inflammation and oxidative damage (P < 0.05). Furthermore, CCP has a therapeutic value in protecting renal function and morphology in diabetic nephropathy by reversing podocyte EMT. The in vitro results indicated that CCP dose-dependently inhibited HG-induced apoptosis, EMT, inflammation, oxidative stress, and mitochondrial ROS levels in MPC5 podocytes (P < 0.05). Luciferase reporter assay confirmed the interaction between miR-30a-3p and TRIM16 in MPC5 podocytes cultured in high glucose (P < 0.05).
Conclusion: The protective effect of CCP on HG-induced MPC5 can be achieved by miR-30a-3p/TRIM16 axis.
Keywords: CCP; Diabetic nephropathy; miR-30a-3p.
© 2023 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures

Similar articles
-
miR-504-3p-HNF1B signaling axis aggravates podocyte injury in diabetic kidney disease.J Mol Histol. 2025 Feb 15;56(2):89. doi: 10.1007/s10735-025-10369-8. J Mol Histol. 2025. PMID: 39954129
-
miR-188-3p abolishes germacrone-mediated podocyte protection in a mouse model of diabetic nephropathy in type I diabetes through triggering mitochondrial injury.Bioengineered. 2022 Jan;13(1):774-788. doi: 10.1080/21655979.2021.2012919. Bioengineered. 2022. Retraction in: Bioengineered. 2024 Dec;15(1):2299558. doi: 10.1080/21655979.2024.2299558. PMID: 34847832 Free PMC article. Retracted.
-
Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis.Int J Biol Macromol. 2020 Nov 15;163:442-456. doi: 10.1016/j.ijbiomac.2020.06.153. Epub 2020 Jun 24. Int J Biol Macromol. 2020. PMID: 32592781
-
Triptolide inhibits oxidative stress and inflammation via the microRNA-155-5p/brain-derived neurotrophic factor to reduce podocyte injury in mice with diabetic nephropathy.Bioengineered. 2022 May;13(5):12275-12288. doi: 10.1080/21655979.2022.2067293. Bioengineered. 2022. PMID: 35603354 Free PMC article.
-
Circ_0000491 Promotes Apoptosis, Inflammation, Oxidative Stress, and Fibrosis in High Glucose-Induced Mesangial Cells by Regulating miR-455-3p/Hmgb1 Axis.Nephron. 2022;146(1):72-83. doi: 10.1159/000516870. Epub 2021 Sep 2. Nephron. 2022. PMID: 34474408
Cited by
-
β-Sitosterol Mitigates Apoptosis, Oxidative Stress and Inflammatory Response by Inactivating TLR4/NF-кB Pathway in Cell Models of Diabetic Nephropathy.Cell Biochem Biophys. 2025 Mar;83(1):1249-1262. doi: 10.1007/s12013-024-01559-4. Epub 2024 Oct 19. Cell Biochem Biophys. 2025. PMID: 39424766
-
Noncoding RNAs and diabetic kidney disease.J Diabetes Investig. 2025 Jan;16(1):8-9. doi: 10.1111/jdi.14331. Epub 2024 Oct 3. J Diabetes Investig. 2025. PMID: 39361944 Free PMC article.
-
Targeting programmed cell death in diabetic kidney disease: from molecular mechanisms to pharmacotherapy.Mol Med. 2024 Dec 20;30(1):265. doi: 10.1186/s10020-024-01020-5. Mol Med. 2024. PMID: 39707216 Free PMC article. Review.
-
The emerging role of E3 ubiquitin ligases and deubiquitinases in metabolic dysfunction-associated steatotic liver disease.J Transl Med. 2025 Mar 25;23(1):368. doi: 10.1186/s12967-025-06255-2. J Transl Med. 2025. PMID: 40133964 Free PMC article. Review.
-
Gut Microbiota and Their Metabolites: The Hidden Driver of Diabetic Nephropathy? Unveiling Gut Microbe's Role in DN.J Diabetes. 2025 Apr;17(4):e70068. doi: 10.1111/1753-0407.70068. J Diabetes. 2025. PMID: 40189872 Free PMC article. Review.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

