Molecularly informed field theory for estimating critical micelle concentrations of intrinsically disordered protein surfactants
- PMID: 38149742
- PMCID: PMC10754628
- DOI: 10.1063/5.0178910
Molecularly informed field theory for estimating critical micelle concentrations of intrinsically disordered protein surfactants
Abstract
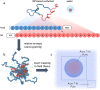
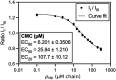
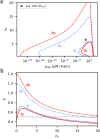
The critical micelle concentration (CMC) is a crucial parameter in understanding the self-assembly behavior of surfactants. In this study, we combine simulation and experiment to demonstrate the predictive capability of molecularly informed field theories in estimating the CMC of biologically based protein surfactants. Our simulation approach combines the relative entropy coarse-graining of small-scale atomistic simulations with large-scale field-theoretic simulations, allowing us to efficiently compute the free energy of micelle formation necessary for the CMC calculation while preserving chemistry-specific information about the underlying surfactant building blocks. We apply this methodology to a unique intrinsically disordered protein platform capable of a wide variety of tailored sequences that enable tunable micelle self-assembly. The computational predictions of the CMC closely match experimental measurements, demonstrating the potential of molecularly informed field theories as a valuable tool to investigate self-assembly in bio-based macromolecules systematically.
© 2023 Author(s). Published under an exclusive license by AIP Publishing.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures