Recent Advances in the Development of Monoclonal Antibodies and Next-Generation Antibodies
- PMID: 38149884
- PMCID: PMC10759153
- DOI: 10.4049/immunohorizons.2300102
Recent Advances in the Development of Monoclonal Antibodies and Next-Generation Antibodies
Abstract
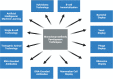
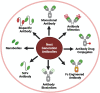
mAbs are highly indispensable tools for diagnostic, prophylactic, and therapeutic applications. The first technique, hybridoma technology, was based on fusion of B lymphocytes with myeloma cells, which resulted in generation of single mAbs against a specific Ag. Along with hybridoma technology, several novel and alternative methods have been developed to improve mAb generation, ranging from electrofusion to the discovery of completely novel technologies such as B cell immortalization; phage, yeast, bacterial, ribosome, and mammalian display systems; DNA/RNA encoded Abs; single B cell technology; transgenic animals; and artificial intelligence/machine learning. This commentary outlines the evolution, methodology, advantages, and limitations of various mAb production techniques. Furthermore, with the advent of next-generation Ab technologies such as single-chain variable fragments, nanobodies, bispecific Abs, Fc-engineered Abs, Ab biosimilars, Ab mimetics, and Ab-drug conjugates, the healthcare and pharmaceutical sectors have become resourceful to develop highly specific mAb treatments against various diseases such as cancer and autoimmune and infectious diseases.
Copyright © 2023 The Authors.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
References
-
- Kulkarni, R. 2020. Antibody-dependent enhancement of viral infections. In Dynamics of Immune Activation in Viral Diseases. Bramhachari P., ed. Springer, Singapore, p. 9–41.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources