A novel, high-performance, low-volume, rapid luciferase immunoprecipitation system (LIPS) assay to detect autoantibodies to zinc transporter 8
- PMID: 38150393
- PMCID: PMC10876106
- DOI: 10.1093/cei/uxad139
A novel, high-performance, low-volume, rapid luciferase immunoprecipitation system (LIPS) assay to detect autoantibodies to zinc transporter 8
Abstract
Background: Zinc transporter 8 autoantibodies (ZnT8A) are thought to appear close to type 1 diabetes (T1D) onset and can identify high-risk multiple (≥2) autoantibody positive individuals. Radiobinding assays (RBA) are widely used for ZnT8A measurement but have limited sustainability. We sought to develop a novel, high-performance, non-radioactive luciferase immunoprecipitation system (LIPS) assay to replace RBA.
Methods: A custom dual C-terminal ZnT8 (aa268-369; R325/W325) heterodimeric antigen, tagged with a NanoluciferaseTM (Nluc-ZnT8) reporter, and LIPS assay was developed. Assay performance was evaluated by testing sera from new onset T1D (n = 573), healthy schoolchildren (n = 521), and selected first-degree relatives (FDRs) from the Bart's Oxford family study (n = 617; 164 progressed to diabetes).
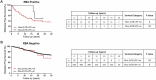
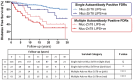
Results: In new-onset T1D, ZnT8A levels by LIPS strongly correlated with RBA (Spearman's r = 0.89; P < 0.0001), and positivity was highly concordant (94.3%). At a high specificity (95%), LIPS and RBA had comparable assay performance [LIPS pROC-AUC(95) 0.032 (95% CI: 0.029-0.036); RBA pROC-AUC(95) 0.031 (95% CI: 0.028-0.034); P = 0.376]. Overall, FDRs found positive by LIPS or RBA had a comparable 20-year diabetes risk (52.6% and 59.7%, respectively), but LIPS positivity further stratified T1D risk in FDRs positive for at least one other islet autoantibody detected by RBA (P = 0.0346).
Conclusion: This novel, high-performance, cheaper, quicker, higher throughput, low blood volume Nluc-ZnT8 LIPS assay is a safe, non-radioactive alternative to RBA with enhanced sensitivity and ability to discriminate T1D progressors. This method offers an advanced approach to current strategies to screen the general population for T1D risk for immunotherapy trials and to reduce rates of diabetic ketoacidosis at diagnosis.
Keywords: LIPS; autoantibodies; immunoassay; method development; risk prediction; type 1 diabetes.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
The authors declare that there are no conflicts of interest associated with this manuscript.
Figures



References
-
- Vermeulen I, Weets I, Asanghanwa M, Ruige J, Van Gaal L, Mathieu C, et al. .; Belgian Diabetes Registry. Contribution of antibodies against IA-2beta and zinc transporter 8 to classification of diabetes diagnosed under 40 years of age. Diabetes Care 2011, 34, 1760–5. doi:10.2337/dc10-2268 - DOI - PMC - PubMed
-
- Williams CL, Aitken RJ, Wilson IV, Mortimer GLM, Long AE, Williams AJK, et al. .; BOX Study Group. The measurement of autoantibodies to insulin informs diagnosis of diabetes in a childhood population negative for other autoantibodies. Diabet Med 2022, 39, e14979. doi:10.1111/dme.14979 - DOI - PMC - PubMed
-
- Gorus FK, Balti EV, Messaaoui A, Demeester S, Van Dalem A, Costa O, et al. .; Belgian Diabetes Registry. Twenty-year progression rate to clinical onset according to autoantibody profile, age, and HLA-DQ genotype in a registry-based group of children and adults with a first-degree relative with type 1 diabetes. Diabetes Care 2017, 40, 1065–72. doi:10.2337/dc16-2228 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

