Standardized viscosity as a source of error in computational fluid dynamic simulations of cerebral aneurysms
- PMID: 38150511
- PMCID: PMC10922831
- DOI: 10.1002/mp.16926
Standardized viscosity as a source of error in computational fluid dynamic simulations of cerebral aneurysms
Abstract
Background: Computational fluid dynamics (CFD) simulations are a powerful tool for studying cerebral aneurysms, capable of evaluating hemodynamics in a way that is infeasible with imaging alone. However, the difficulty of incorporating patient-specific information and inherent obstacles of in vivo validation have limited the clinical usefulness of CFD of cerebral aneurysms. In this work we investigate the effect of using standardized blood viscosity values in CFD simulations of cerebral aneurysms when compared to simulations of the same aneurysms using patient-specific viscosity values derived from hematocrit measurements.
Purpose: The objective of this work is to determine the level of error, on average, that is, caused by using standardized values of viscosity in CFD simulations of cerebral aneurysms. By quantifying this error, we demonstrate the need for incorporating patient-specific viscosity in future CFD investigations of cerebral aneurysms.
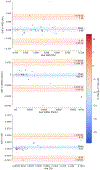
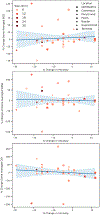
Methods: CFD simulations of forty-one cerebral aneurysms were conducted using patient-specific boundary conditions. For each aneurysm two simulations were conducted, one utilizing patient-specific blood viscosity derived from hematocrit measurements and another using a standardized value for blood viscosity. Hemodynamic parameters such as wall shear stress (WSS), wall shear stress gradient (WSSG), and the oscillatory shear index (OSI) were calculated for each of the simulations for each aneurysm. Paired t-tests for differences in the time-averaged maps of these hemodynamic parameters between standardized and patient-specific viscosity simulations were conducted for each aneurysm. Bland-Altman analysis was used to examine the cohort-wide changes in the hemodynamic parameters. Subjects were broken into two groups, those with higher than standard viscosity and those with lower than standard viscosity. An unpaired t-test was used to compare the percent change in WSS, WSSG, and OSI between patient-specific and standardized viscosity simulations for the two cohorts. The percent changes in hemodynamic parameters were correlated against the direction and magnitude of percent change in viscosity, aneurysm size, and aneurysm location. For all t-tests, a Bonferroni-corrected significance level of 0.0167 was used.
Results: 63.2%, 41.5%, and 48.7% of aneurysms showed statistically significant differences between patient-specific and standardized viscosity simulations for WSS, WSSG, and OSI respectively. No statistically significant difference was found in the percent changes in WSS, WSSG, and OSI between the group with higher than standard viscosity and those with lower than standard viscosity, indicating an increase in viscosity can cause either an increase or decrease in each of the hemodynamic parameters. On a study-wide level no significant bias was found in either direction for WSS, WSSG, or OSI between the simulation groups due to the bidirectional effect of changing viscosity. No correlation was found between percent change of viscosity and percent change of WSS, WSSG, or OSI, meaning an after-the-fact correction for patient-specific viscosity is not feasible.
Conclusion: Standardizing viscosity values in CFD of cerebral aneurysms has a large and unpredictable impact on the calculated WSS, WSSG, and OSI when compared to CFD simulations of the same aneurysms using a patient-specific viscosity. We recommend implementing hematocrit-based patient-specific blood viscosity values for all CFD simulations of cerebral aneurysms.
Keywords: cerebral aneurysm; computational fluid dynamics; viscosity.
© 2023 American Association of Physicists in Medicine.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors have no relevant conflicts of interest to disclose.
Figures


Similar articles
-
Wall shear stress gradient is independently associated with middle cerebral artery aneurysm development: a case-control CFD patient-specific study based on 77 patients.BMC Neurol. 2021 Jul 19;21(1):281. doi: 10.1186/s12883-021-02251-3. BMC Neurol. 2021. PMID: 34281533 Free PMC article.
-
The effect of inlet waveforms on computational hemodynamics of patient-specific intracranial aneurysms.J Biomech. 2014 Dec 18;47(16):3882-90. doi: 10.1016/j.jbiomech.2014.09.034. Epub 2014 Oct 13. J Biomech. 2014. PMID: 25446264 Free PMC article.
-
Impact of blood viscosity on hemodynamics of large intracranial aneurysms.Clin Neurol Neurosurg. 2024 Nov;246:108543. doi: 10.1016/j.clineuro.2024.108543. Epub 2024 Sep 11. Clin Neurol Neurosurg. 2024. PMID: 39265483
-
Computational Fluid Dynamics for Cerebral Aneurysms in Clinical Settings.Acta Neurochir Suppl. 2021;132:27-32. doi: 10.1007/978-3-030-63453-7_4. Acta Neurochir Suppl. 2021. PMID: 33973025 Review.
-
Computational fluid dynamics as a risk assessment tool for aneurysm rupture.Neurosurg Focus. 2019 Jul 1;47(1):E12. doi: 10.3171/2019.4.FOCUS19189. Neurosurg Focus. 2019. PMID: 31261116 Review.
Cited by
-
Computational fluid dynamics analysis for predicting microaneurysm formation in parent arteries of unruptured cerebral aneurysms: implications for neck clipping safety.Front Neurol. 2025 Mar 3;16:1531703. doi: 10.3389/fneur.2025.1531703. eCollection 2025. Front Neurol. 2025. PMID: 40098687 Free PMC article.
References
-
- Suzuki T, Genkai N, Nomura T, Abe H. Assessing the hemodynamics in residual cavities of intracranial aneurysm after coil embolization with combined computational flow dynamics and silent magnetic resonance angiography. J Stroke Cerebrovasc Dis. 2020;29(12):105290–105290. doi:10.1016/j.jstrokecerebrovasdis.2020.105290 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

