Hemopexin reverses activation of lung eIF2α and decreases mitochondrial injury in chlorine-exposed mice
- PMID: 38150547
- PMCID: PMC11281818
- DOI: 10.1152/ajplung.00273.2023
Hemopexin reverses activation of lung eIF2α and decreases mitochondrial injury in chlorine-exposed mice
Abstract
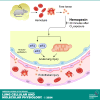
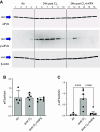
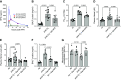
We assessed the mechanisms by which nonencapsulated heme, released in the plasma of mice after exposure to chlorine (Cl2) gas, resulted in the initiation and propagation of acute lung injury. We exposed adult male and female C57BL/6 mice to Cl2 (500 ppm for 30 min), returned them to room air, and injected them intramuscularly with either human hemopexin (hHPX; 5 µg/g BW in 50-µL saline) or vehicle at 1 h post-exposure. Upon return to room air, Cl2-exposed mice, injected with vehicle, developed respiratory acidosis, increased concentrations of plasma proteins in the alveolar space, lung mitochondrial DNA injury, increased levels of free plasma heme, and major alterations of their lung proteome. hHPX injection mice mitigated the onset and development of lung and mitochondrial injury and the increase of plasma heme, reversed the Cl2-induced changes in 83 of 237 proteins in the lung proteome at 24 h post-exposure, and improved survival at 15 days post-exposure. Systems biology analysis of the lung global proteomics data showed that hHPX reversed changes in a number of key pathways including elF2 signaling, verified by Western blotting measurements. Recombinant human hemopexin, generated in tobacco plants, injected at 1 h post-Cl2 exposure, was equally effective in reversing acute lung and mtDNA injury. The results of this study offer new insights as to the mechanisms by which exposure to Cl2 results in acute lung injury and the therapeutic effects of hemopexin.NEW & NOTEWORTHY Herein, we demonstrate that exposure of mice to chlorine gas causes significant changes in the lung proteome 24 h post-exposure. Systems biology analysis of the proteomic data is consistent with damage to mitochondria and activation of eIF2, the master regulator of transcription and protein translation. Post-exposure injection of hemopexin, which scavenges free heme, attenuated mtDNA injury, eIF2α phosphorylation, decreased lung injury, and increased survival.
Keywords: lung injury; mitochondrial DNA; proteomics; recombinant hemopexin.
Conflict of interest statement
S. Matalon and T. Jilling are Inventors in a US Provisional Patent Application #62/896,427, “Use of Hemopexin as a Treatment for Pulmonary Injury” [filed September 5, 2019; Inventors: Dr. Sadis Matalon (Primary), Dr. Saurabh Aggarwal, Dr. Tamas Jilling, Dr. Rakesh Patel]. S. Matalon is the Editor in Chief of
Figures

Update of
-
Hemopexin Reverses Activation of Lung eIF2a and Decreases Mitochondrial Injury in Chlorine Exposed Mice.bioRxiv [Preprint]. 2023 Aug 19:2023.08.17.553717. doi: 10.1101/2023.08.17.553717. bioRxiv. 2023. Update in: Am J Physiol Lung Cell Mol Physiol. 2024 Apr 1;326(4):L440-L457. doi: 10.1152/ajplung.00273.2023. PMID: 37645744 Free PMC article. Updated. Preprint.
Similar articles
-
Oxidative damage to lung mitochondrial DNA is a key contributor to the development of chemical lung injury.Redox Biol. 2025 May;82:103624. doi: 10.1016/j.redox.2025.103624. Epub 2025 Mar 29. Redox Biol. 2025. PMID: 40209617 Free PMC article.
-
Hemopexin Reverses Activation of Lung eIF2a and Decreases Mitochondrial Injury in Chlorine Exposed Mice.bioRxiv [Preprint]. 2023 Aug 19:2023.08.17.553717. doi: 10.1101/2023.08.17.553717. bioRxiv. 2023. Update in: Am J Physiol Lung Cell Mol Physiol. 2024 Apr 1;326(4):L440-L457. doi: 10.1152/ajplung.00273.2023. PMID: 37645744 Free PMC article. Updated. Preprint.
-
Chlorine inhalation induces acute chest syndrome in humanized sickle cell mouse model and ameliorated by postexposure hemopexin.Redox Biol. 2021 Aug;44:102009. doi: 10.1016/j.redox.2021.102009. Epub 2021 May 17. Redox Biol. 2021. PMID: 34044323 Free PMC article.
-
Chlorine-induced cardiopulmonary injury.Ann N Y Acad Sci. 2016 Jun;1374(1):159-67. doi: 10.1111/nyas.13091. Epub 2016 Jun 15. Ann N Y Acad Sci. 2016. PMID: 27303906 Free PMC article. Review.
-
Emergency management of chlorine gas exposure - a systematic review.Clin Toxicol (Phila). 2019 Feb;57(2):77-98. doi: 10.1080/15563650.2018.1519193. Epub 2019 Jan 23. Clin Toxicol (Phila). 2019. PMID: 30672349
Cited by
-
scFocus: Detecting branching probabilities in single-cell data with SAC.Comput Struct Biotechnol J. 2025 May 20;27:2243-2263. doi: 10.1016/j.csbj.2025.04.036. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40520594 Free PMC article.
-
Oxidative damage to lung mitochondrial DNA is a key contributor to the development of chemical lung injury.Redox Biol. 2025 May;82:103624. doi: 10.1016/j.redox.2025.103624. Epub 2025 Mar 29. Redox Biol. 2025. PMID: 40209617 Free PMC article.
-
The long road to Ithaca: a physiologist's journey.Am J Physiol Cell Physiol. 2025 May 1;328(5):C1526-C1534. doi: 10.1152/ajpcell.00030.2025. Epub 2025 Feb 24. Am J Physiol Cell Physiol. 2025. PMID: 39993005 Free PMC article. Review.
References
-
- Alishlash AS, Sapkota M, Ahmad I, Maclin K, Ahmed NA, Molyvdas A, Doran S, Albert CJ, Aggarwal S, Ford DA, Ambalavanan N, Jilling T, Matalon S. Chlorine inhalation induces acute chest syndrome in humanized sickle cell mouse model and ameliorated by post-exposure hemopexin. Redox Biol 44: 102009, 2021. doi:10.1016/j.redox.2021.102009. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R21 ES032956/ES/NIEHS NIH HHS/United States
- 5R2 ES031559/HHS | NIH | National Institute of Environmental Health Sciences (NIEHS)
- 1R21ES034226;/HHS | NIH | National Institute of Environmental Health Sciences (NIEHS)
- R21 ES031559/ES/NIEHS NIH HHS/United States
- 2R01HL031197-28/HHS | NIH | NHLBI | NHLBI Division of Intramural Research (DIR)
LinkOut - more resources
Full Text Sources

