Crystal Structure, Steady-State, and Pre-Steady-State Kinetics of Acinetobacter baumannii ATP Phosphoribosyltransferase
- PMID: 38150593
- PMCID: PMC10795190
- DOI: 10.1021/acs.biochem.3c00551
Crystal Structure, Steady-State, and Pre-Steady-State Kinetics of Acinetobacter baumannii ATP Phosphoribosyltransferase
Abstract
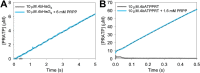
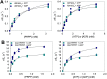
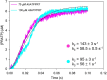
The first step of histidine biosynthesis in Acinetobacter baumannii, the condensation of ATP and 5-phospho-α-d-ribosyl-1-pyrophosphate to produce N1-(5-phospho-β-d-ribosyl)-ATP (PRATP) and pyrophosphate, is catalyzed by the hetero-octameric enzyme ATP phosphoribosyltransferase, a promising target for antibiotic design. The catalytic subunit, HisGS, is allosterically activated upon binding of the regulatory subunit, HisZ, to form the hetero-octameric holoenzyme (ATPPRT), leading to a large increase in kcat. Here, we present the crystal structure of ATPPRT, along with kinetic investigations of the rate-limiting steps governing catalysis in the nonactivated (HisGS) and activated (ATPPRT) forms of the enzyme. A pH-rate profile showed that maximum catalysis is achieved above pH 8.0. Surprisingly, at 25 °C, kcat is higher when ADP replaces ATP as substrate for ATPPRT but not for HisGS. The HisGS-catalyzed reaction is limited by the chemical step, as suggested by the enhancement of kcat when Mg2+ was replaced by Mn2+, and by the lack of a pre-steady-state burst of product formation. Conversely, the ATPPRT-catalyzed reaction rate is determined by PRATP diffusion from the active site, as gleaned from a substantial solvent viscosity effect. A burst of product formation could be inferred from pre-steady-state kinetics, but the first turnover was too fast to be directly observed. Lowering the temperature to 5 °C allowed observation of the PRATP formation burst by ATPPRT. At this temperature, the single-turnover rate constant was significantly higher than kcat, providing additional evidence for a step after chemistry limiting catalysis by ATPPRT. This demonstrates allosteric activation by HisZ accelerates the chemical step.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

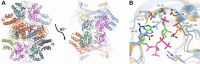




References
-
- Ceparano M.; Baccolini V.; Migliara G.; Isonne C.; Renzi E.; Tufi D.; De Vito C.; De Giusti M.; Trancassini M.; Alessandri F.; Ceccarelli G.; Pugliese F.; Villari P.; Angiulli M.; Battellito S.; Bellini A.; Bongiovanni A.; Caivano L.; Castellani M.; Coletti M.; Cottarelli A.; D’Agostino L.; De Giorgi A.; De Marchi C.; Germani I.; Giannini D.; Mazzeo E.; Orlandi S.; Piattoli M.; Ricci E.; Siena L. M.; Territo A.; Vrenna G.; Zanni S.; Marzuillo C. Acinetobacter baumannii isolates from COVID-19 patients in a hospital intensive care unit: Molecular typing and risk factors. Microorganisms 2022, 10, 722. 10.3390/microorganisms10040722. - DOI - PMC - PubMed
-
- Tacconelli E.; Carrara E.; Savoldi A.; Harbarth S.; Mendelson M.; Monnet D. L.; Pulcini C.; Kahlmeter G.; Kluytmans J.; Carmeli Y.; Ouellette M.; Outterson K.; Patel J.; Cavaleri M.; Cox E. M.; Houchens C. R.; Grayson M. L.; Hansen P.; Singh N.; Theuretzbacher U.; Magrini N.; Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
-
- Huang Y.; Zhou Q.; Wang W.; Huang Q.; Liao J.; Li J.; Long L.; Ju T.; Zhang Q.; Wang H.; Xu H.; Tu M. Acinetobacter baumannii ventilator-associated pneumonia: Clinical efficacy of combined antimicrobial therapy and in vitro drug sensitivity test results. Front. Pharmacol. 2019, 10, 92. 10.3389/fphar.2019.00092. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

