Immune Checkpoint-Blocking Nanocages Cross the Blood-Brain Barrier and Impede Brain Tumor Growth
- PMID: 38150627
- PMCID: PMC10777349
- DOI: 10.1021/acsbiomaterials.3c01200
Immune Checkpoint-Blocking Nanocages Cross the Blood-Brain Barrier and Impede Brain Tumor Growth
Abstract
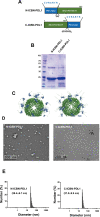
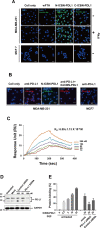
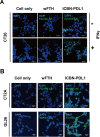
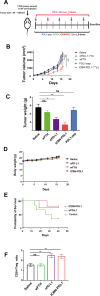
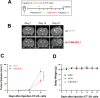
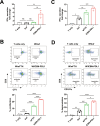
Glioblastoma (GBM) is the deadliest tumor of the central nervous system, with a median survival of less than 15 months. Despite many trials, immune checkpoint-blocking (ICB) therapies using monoclonal antibodies against the PD-1/PD-L1 axis have demonstrated only limited benefits for GBM patients. Currently, the main hurdles in brain tumor therapy include limited drug delivery across the blood-brain barrier (BBB) and the profoundly immune-suppressive microenvironment of GBM. Thus, there is an urgent need for new therapeutics that can cross the BBB and target brain tumors to modulate the immune microenvironment. To this end, we developed an ICB strategy based on the BBB-permeable, 24-subunit human ferritin heavy chain, modifying the ferritin surface with 24 copies of PD-L1-blocking peptides to create ferritin-based ICB nanocages. The PD-L1pep ferritin nanocages first demonstrated their tumor-targeting and antitumor activities in an allograft colon cancer model. Next, we found that these PD-L1pep ferritin nanocages efficiently penetrated the BBB and targeted brain tumors through specific interactions with PD-L1, significantly inhibiting tumor growth in an orthotopic intracranial tumor model. The addition of PD-L1pep ferritin nanocages to triple in vitro cocultures of T cells, GBM cells, and glial cells significantly inhibited PD-1/PD-L1 interactions and restored T-cell activity. Collectively, these findings indicate that ferritin nanocages displaying PD-L1-blocking peptides can overcome the primary hurdle of brain tumor therapy and are, therefore, promising candidates for treating GBM.
Keywords: PD-L1 binding peptide; blood brain barrier; ferritin; glioblastoma; immune checkpoint; nanocage.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Application prospect of peptide-modified nano targeting drug delivery system combined with PD-1/PD-L1 based immune checkpoint blockade in glioblastoma.Int J Pharm. 2020 Nov 15;589:119865. doi: 10.1016/j.ijpharm.2020.119865. Epub 2020 Sep 9. Int J Pharm. 2020. PMID: 32919004 Review.
-
Anticancer nanocage platforms for combined immunotherapy designed to harness immune checkpoints and deliver anticancer drugs.Biomaterials. 2021 Mar;270:120685. doi: 10.1016/j.biomaterials.2021.120685. Epub 2021 Jan 22. Biomaterials. 2021. PMID: 33524811
-
Current advances in PD-1/PD-L1 axis-related tumour-infiltrating immune cells and therapeutic regimens in glioblastoma.Crit Rev Oncol Hematol. 2020 Jul;151:102965. doi: 10.1016/j.critrevonc.2020.102965. Epub 2020 Apr 24. Crit Rev Oncol Hematol. 2020. PMID: 32442903 Review.
-
Allomelanin-based biomimetic nanotherapeutics for orthotopic glioblastoma targeted photothermal immunotherapy.Acta Biomater. 2023 Aug;166:552-566. doi: 10.1016/j.actbio.2023.05.037. Epub 2023 May 24. Acta Biomater. 2023. PMID: 37236575
-
Targeting the PD-1/PD-L1 pathway in glioblastoma multiforme: Preclinical evidence and clinical interventions.Int Immunopharmacol. 2021 Apr;93:107403. doi: 10.1016/j.intimp.2021.107403. Epub 2021 Feb 12. Int Immunopharmacol. 2021. PMID: 33581502 Review.
Cited by
-
Research progress and challenges of the PD-1/PD-L1 axis in gliomas.Cell Biosci. 2024 Sep 27;14(1):123. doi: 10.1186/s13578-024-01305-6. Cell Biosci. 2024. PMID: 39334448 Free PMC article. Review.
-
Design and application of ferritin-based nanomedicine for targeted cancer therapy.Nanomedicine (Lond). 2025 Mar;20(5):481-500. doi: 10.1080/17435889.2025.2459056. Epub 2025 Feb 3. Nanomedicine (Lond). 2025. PMID: 39895329 Review.
-
Protein-based nanoparticles for antimicrobial and cancer therapy: implications for public health.RSC Adv. 2025 May 8;15(19):14966-15016. doi: 10.1039/d5ra01427a. eCollection 2025 May 6. RSC Adv. 2025. PMID: 40343307 Free PMC article. Review.
References
-
- Wei S. C.; Duffy C. R.; Allison J. P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 2018, 8 (9), 1069–1086. 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
