Importance of NK Cells in Cellular and Humoral Responses Triggered by Pneumococcus Vaccination
- PMID: 38151005
- PMCID: PMC11126198
- DOI: 10.1159/000535562
Importance of NK Cells in Cellular and Humoral Responses Triggered by Pneumococcus Vaccination
Abstract
Introduction: Despite the success of vaccination in reducing overall rate of pneumococcal pneumonia, Streptococcus pneumoniae is still held responsible for high mortality and modality rates worldwide. Our study aimed to investigate the potential role played by NK cells in immune response generated by pneumococcal vaccination, which could contribute to the development of more effective vaccines.
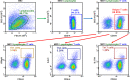
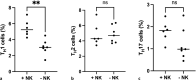
Methods: The study included mice with and without NK cell depletion which were immunized with pneumococcus polysaccharide-conjugated vaccine followed by pneumococcus polysaccharide vaccine (PPV). Serum samples and splenocytes were collected from mice sacrificed 4 weeks after the last PPV dose. Serum samples were used for antibody level quantification by ELISA assay, while splenocytes were treated with PPV in vitro before monitoring CD4+ T-cell subsets (TH1, TH2, and TH17) and cytokine (IFN-γ, IL-4, and IL-17) secretion levels by flow cytometry and ELISA analysis, respectively.
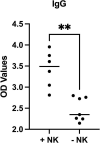
Results: Results demonstrated reduced pneumococcal IgG and TH1 cell levels due to NK cell depletion. Nevertheless, in contrast to these observations, IFN-γ secretion levels after in vitro PPV-23 treatment of splenocytes did not exhibit any statistically significant difference between the two mice groups.
Conclusions: The data indicate a positive contribution of NK cells to both T-cell and B-cell responses triggered against pneumococcal vaccination. Further studies are required to confirm our data and investigate the potential benefit of NK cell targeting in promoting vaccine efficacy, especially in the elderly population who continues to be affected significantly by pneumococcal pneumonia.
Keywords: IgG; NK cells; Streptococcus pneumoniae; TH1; Vaccination.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Vaccination With the Commensal Streptococcus mitis Expressing Pneumococcal Serotype 5 Capsule Elicits IgG/IgA and Th17 Responses Against Streptococcus pneumoniae.Front Immunol. 2021 Apr 19;12:676488. doi: 10.3389/fimmu.2021.676488. eCollection 2021. Front Immunol. 2021. PMID: 33953733 Free PMC article.
-
Th17/Th1 biased immunity to the pneumococcal proteins PcsB, StkP and PsaA in adults of different age.Vaccine. 2011 May 23;29(23):3982-9. doi: 10.1016/j.vaccine.2011.03.081. Epub 2011 Apr 8. Vaccine. 2011. PMID: 21481328
-
Pneumococcal Conjugate Vaccine Does Not Induce Humoral Response When Administrated Within the Six Months After CD19 CAR T-Cell Therapy.Transplant Cell Ther. 2023 Apr;29(4):277.e1-277.e9. doi: 10.1016/j.jtct.2022.08.011. Epub 2022 Aug 12. Transplant Cell Ther. 2023. PMID: 35970303
-
Experience with pneumococcal polysaccharide conjugate vaccine (conjugated to CRM197 carrier protein) in children and adults.Clin Microbiol Infect. 2013 Oct;19 Suppl 1:1-9. doi: 10.1111/1469-0691.12320. Clin Microbiol Infect. 2013. PMID: 24083785 Review.
-
The role of vaccination in preventing pneumococcal disease in adults.Clin Microbiol Infect. 2014 May;20 Suppl 5(0 5):52-8. doi: 10.1111/1469-0691.12518. Epub 2014 Feb 22. Clin Microbiol Infect. 2014. PMID: 24410778 Free PMC article. Review.
References
-
- Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, et al. . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018:18. - PMC - PubMed
-
- Golos M, Eliakim-Raz N, Stern A, Leibovici L, Paul M. Conjugated pneumococcal vaccine versus polysaccharide pneumococcal vaccine for prevention of pneumonia and invasive pneumococcal disease in immunocompetent and immunocompromised adults and children. Cochrane Database Syst Rev. 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

