Fucoxanthin diminishes oxidative stress damage in human placenta-derived mesenchymal stem cells through the PI3K/Akt/Nrf-2 pathway
- PMID: 38151503
- PMCID: PMC10752906
- DOI: 10.1038/s41598-023-49751-5
Fucoxanthin diminishes oxidative stress damage in human placenta-derived mesenchymal stem cells through the PI3K/Akt/Nrf-2 pathway
Abstract
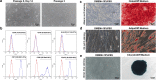
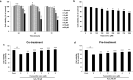
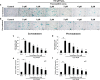
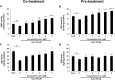
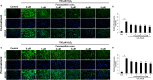
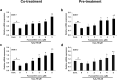
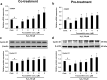
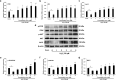
Placenta-derived mesenchymal stem cells (PL-MSCs) have therapeutic potential in various clinical contexts due to their regenerative and immunomodulatory properties. However, with increasing age or extensive in vitro culture, their viability and function are gradually lost, thus restricting their therapeutic application. The primary cause of this deterioration is oxidative injury from free radicals. Therefore, enhancing cell viability and restoring cellular repair mechanisms of PL-MSCs in an oxidative stress environment are crucial in this context. Fucoxanthin, a carotenoid derived from brown seaweed, demonstrates antioxidant activity by increasing the production of antioxidant enzymes and lowering the levels of reactive oxygen species (ROS). This study aimed to determine whether fucoxanthin protects PL-MSCs from hydrogen peroxide (H2O2)-induced oxidative stress. After characterization, PL-MSCs were co-treated with fucoxanthin and H2O2 for 24 h (co-treatment) or pre-treated with fucoxanthin for 24 h followed by H2O2 for 24 h (pre-treatment). The effects of fucoxanthin on cell viability and proliferation were examined using an MTT assay. The expression of antioxidant enzymes, PI3K/Akt/Nrf-2 and intracellular ROS production were investigated in fucoxanthin-treated PL-MSCs compared to the untreated group. The gene expression and involvement of specific pathways in the cytoprotective effect of fucoxanthin were investigated by high-throughput NanoString nCounter analysis. The results demonstrated that co-treatment and pre-treatment with fucoxanthin restored the viability and proliferative capacity of PL-MSCs. Fucoxanthin treatment increased the expression of antioxidant enzymes in PL-MSCs cultured under oxidative stress conditions and decreased intracellular ROS accumulation. Markedly, fucoxanthin treatment could restore PI3K/Akt/Nrf-2 expression in H2O2-treated PL-MSCs. High-throughput analysis revealed up-regulation of genes involved in cell survival pathways, including cell cycle and proliferation, DNA damage repair pathways, and down-regulation of genes in apoptosis and autophagy pathways. This study demonstrated that fucoxanthin protects and rescues PL-MSCs from oxidative stress damage through the PI3K/Akt/Nrf-2 pathway. Our data provide the supporting evidence for the use of fucoxanthin as an antioxidant cytoprotective agent to improve the viability and proliferation capacity of PL-MSCs both in vitro and in vivo required to increase the effectiveness of MSC expansion for therapeutic applications.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Selenomethionine protects oxidative-stress-damaged bone-marrow-derived mesenchymal stem cells via an antioxidant effect and the PTEN/PI3K/AKT pathway.Exp Cell Res. 2021 Nov 15;408(2):112864. doi: 10.1016/j.yexcr.2021.112864. Epub 2021 Oct 6. Exp Cell Res. 2021. PMID: 34626586
-
High density lipoprotein protects mesenchymal stem cells from oxidative stress-induced apoptosis via activation of the PI3K/Akt pathway and suppression of reactive oxygen species.Int J Mol Sci. 2012 Dec 13;13(12):17104-20. doi: 10.3390/ijms131217104. Int J Mol Sci. 2012. PMID: 23443132 Free PMC article.
-
Induction of Apoptosis in Human Glioma Cells by Fucoxanthin via Triggering of ROS-Mediated Oxidative Damage and Regulation of MAPKs and PI3K-AKT Pathways.J Agric Food Chem. 2019 Feb 27;67(8):2212-2219. doi: 10.1021/acs.jafc.8b07126. Epub 2019 Feb 13. J Agric Food Chem. 2019. PMID: 30688446
-
N-acetylserotonin protects PC12 cells from hydrogen peroxide induced damage through ROS mediated PI3K / AKT pathway.Cell Cycle. 2022 Nov;21(21):2268-2282. doi: 10.1080/15384101.2022.2092817. Epub 2022 Jun 26. Cell Cycle. 2022. PMID: 35758219 Free PMC article. Review.
-
In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments : In vitro augmentation of mesenchymal stem cells viability.Cell Stress Chaperones. 2015 Mar;20(2):237-51. doi: 10.1007/s12192-014-0560-1. Epub 2014 Dec 20. Cell Stress Chaperones. 2015. PMID: 25527070 Free PMC article. Review.
Cited by
-
Tisochrysis lutea Fucoxanthin Suppresses NF-κB, JNK, and p38-Associated MMP Expression in Arthritis Pathogenesis via Antioxidant Activity.Antioxidants (Basel). 2024 Aug 2;13(8):941. doi: 10.3390/antiox13080941. Antioxidants (Basel). 2024. PMID: 39199188 Free PMC article.
-
Fucoxanthin Induces Ferroptosis in Cancer Cells via Downregulation of the Nrf2/HO-1/GPX4 Pathway.Molecules. 2024 Jun 14;29(12):2832. doi: 10.3390/molecules29122832. Molecules. 2024. PMID: 38930897 Free PMC article.
-
Fucoxanthin Inhibits the Proliferation and Metastasis of Human Pharyngeal Squamous Cell Carcinoma by Regulating the PI3K/Akt/mTOR Signaling Pathway.Molecules. 2024 Jul 30;29(15):3603. doi: 10.3390/molecules29153603. Molecules. 2024. PMID: 39125009 Free PMC article.
-
The dual role of mesenchymal stem cells in apoptosis regulation.Cell Death Dis. 2024 Apr 6;15(4):250. doi: 10.1038/s41419-024-06620-x. Cell Death Dis. 2024. PMID: 38582754 Free PMC article. Review.
-
High glucose inhibits proliferation, migration, and osteogenic differentiation of human placenta-derived mesenchymal stem cells.Sci Rep. 2025 Jul 2;15(1):22512. doi: 10.1038/s41598-025-06454-3. Sci Rep. 2025. PMID: 40594898 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

