Trichostatin A enhances the titanium rods osseointegration in osteoporotic rats by the inhibition of oxidative stress through activating the AKT/Nrf2 pathway
- PMID: 38151509
- PMCID: PMC10752907
- DOI: 10.1038/s41598-023-50108-1
Trichostatin A enhances the titanium rods osseointegration in osteoporotic rats by the inhibition of oxidative stress through activating the AKT/Nrf2 pathway
Abstract
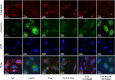
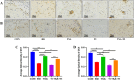
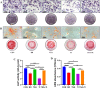
The use of titanium implants as fixed supports following fractures in patients with OP can often result in sterile loosening and poor osseointegration. Oxidative stress has been shown to play a particularly important role in this process. While TSA has been reported to facilitate in vivo osteogenesis, the underlying mechanisms remain to be clarified. It also remains unclear whether TSA can improve the osseointegration of titanium implants. This study investigated whether TSA could enhance the osseointegration of titanium rods by activating AKT/Nrf2 pathway signaling, thereby suppressing oxidative stress. MC3T3-E1 cells treated with CCCP to induce oxidative stress served as an in vitro model, while an OVX-induced OP rat model was employed for in vivo analysis of titanium rod implantation. In vitro, TSA treatment of CCCP-treated MC3T3-E1 cells resulted in the upregulation of osteogenic proteins together with increased AKT, total Nrf2, nuclear Nrf2, HO-1, and NQO1 expression, enhanced mitochondrial functionality, and decreased oxidative damage. Notably, the PI3K/AKT inhibitor LY294002 reversed these effects. In vivo, TSA effectively enhanced the microstructural characteristics of distal femur trabecular bone, increased BMSCs mineralization capacity, promoted bone formation, and improved the binding of titanium implants to the surrounding tissue. Finally, our results showed that TSA could reverse oxidative stress-induced cell damage while promoting bone healing and improving titanium rods' osseointegration through AKT/Nrf2 pathway activation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









References
MeSH terms
Substances
Grants and funding
- YR202226/Talented Scholars of Wannan Medical College
- PF2019005/Technology Mountaineering Program of Yijishan Hospital, and Wannan Medical College
- 2022jc61/Science and Technology Plan Projects of Wu Hu City
- 2023AH040265/Anhui Province Higher Education Science Research Project
- 202304295107020007/Anhui Clinical Medical Research Transformation Project
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

