A multiplexed immunochemical microarray for the determination of cardiovascular disease biomarkers
- PMID: 38151630
- PMCID: PMC10752916
- DOI: 10.1007/s00604-023-06119-w
A multiplexed immunochemical microarray for the determination of cardiovascular disease biomarkers
Abstract
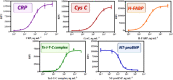
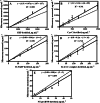
A fluorescence antibody microarray has been developed for the determination of relevant cardiovascular disease biomarkers for the analysis of human plasma samples. Recording characteristic protein molecular fingerprints to assess individual's states of health could allow diagnosis to go beyond the simple identification of the disease, providing information on its stage or prognosis. Precisely, cardiovascular diseases (CVDs) are complex disorders which involve different degenerative processes encompassing a collection of biomarkers related to disease progression or stage. The novel approach that we propose is a fluorescent microarray chip has been developed accomplishing simultaneous determination of the most significant cardiac biomarkers in plasma aiming to determine the CVD status stage of the patient. As proof of concept, we have chosen five relevant biomarkers, C-reactive protein (CRP) as biomarker of inflammation, cystatin C (CysC) as biomarker of renal failure that is directly related with heart failure, cardiac troponin I (cTnI) as already established biomarker for cardiac damage, heart fatty acid binding protein as biomarker of ischemia (H-FABP), and finally, NT-proBNP (N-terminal pro-brain natriuretic peptide), a well-established heart failure biomarker. After the optimization of the multiplexed microarray, the assay allowed the simultaneous determination of 5 biomarkers in a buffer solution reaching LODs of 15 ± 5, 3 ± 1, 24 ± 3, 25 ± 3, and 3 ± 1 ng mL-1, for CRP, CysC, H-FABP, cTnI, and NT-proBNP, respectively. After solving the matrix effect, and demonstrating the accuracy for each biomarker, the chip was able to determine 24 samples per microarray chip. Then, the microarray has been used on a small pilot clinical study with 29 plasma samples from clinical patients which suffered different CVD and other related disorders. Results show the superior capability of the chip to provide clinical information related to the disease in terms of turnaround time (1 h 30 min total assay and measurement) and amount of information delivered in respect to reference technologies used in hospital laboratories (clinical analyzers). Despite the failure to detect c-TnI at the reported threshold, the microarray technology could be a powerful approach to diagnose the cardiovascular disease at early stage, monitor its progress, and eventually providing information about an eminent potential risk of suffering a myocardial infarction. The microarray chip here reported could be the starting point for achieving powerful multiplexed diagnostic technologies for the diagnosis of CVDs or any other pathology for which biomarkers have been identified at different stages of the disease.
Keywords: Acute myocardial infarction; Fluorescence detection; Heart failure; Immunoassay; Inflammation fluorescence; Microarray; Multiplexation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO: http://www.who.int/cardiovascular_diseases/en/. Accessed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
