TREM2-IGF1 Mediated Glucometabolic Enhancement Underlies Microglial Neuroprotective Properties During Ischemic Stroke
- PMID: 38151703
- PMCID: PMC10933614
- DOI: 10.1002/advs.202305614
TREM2-IGF1 Mediated Glucometabolic Enhancement Underlies Microglial Neuroprotective Properties During Ischemic Stroke
Abstract
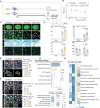
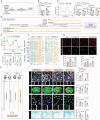
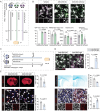
Microglia, the major resident immune cells in the central nervous system, serve as the frontline soldiers against cerebral ischemic injuries, possibly along with metabolic alterations. However, signaling pathways involved in the regulation of microglial immunometabolism in ischemic stroke remain to be further elucidated. In this study, using single-nuclei RNA sequencing, a microglial subcluster up-regulated in ischemic brain tissues is identified, with high expression of Igf1 and Trem2, neuroprotective transcriptional signature and enhanced oxidative phosphorylation. Microglial depletion by PLX3397 exacerbates ischemic brain damage, which is reversed by repopulating the microglia with high Igf1 and Trem2 phenotype. Mechanistically, Igf1 serves as one of the major down-stream molecules of Trem2, and Trem2-Igf1 signaling axis regulates microglial functional and metabolic profiles, exerting neuroprotective effects on ischemic stroke. Overexpression of Igf1 and supplementation of cyclocreatine restore microglial glucometabolic levels and cellular functions even in the absence of Trem2. These findings suggest that Trem2-Igf1 signaling axis reprograms microglial immunometabolic profiles and shifts microglia toward a neuroprotective phenotype, which has promising therapeutic potential in treating ischemic stroke.
Keywords: Trem2-Igf1 signaling pathway; ischemic stroke; microglia; oxidative phosphorylation.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Montaner J., Ramiro L., Simats A., Tiedt S., Makris K., Jickling G. C., Debette S., Sanchez J.‐C., Bustamante A., Nat. Rev. Neurol. 2020, 16, 247. - PubMed
-
- Monsorno K., Buckinx A.n, Paolicelli R. C., Trends Endocrinol. Metab. 2022, 33, 186. - PubMed
-
- Van Den Bossche J., O'neill L. A., Menon D., Trends Immunol. 2017, 38, 395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022ZD0204704/Ministry of Science and Technology China Brain Initiative
- 2022020801020454/Knowledge Innovation Program of Wuhan Shuguang Project
- 2020YQ06/Tongji Hospital (HUST) Foundation for Excellent Young Scientist
- 82371404/Innovative Research Group Project of the National Natural Science Foundation of China
- 82071380/Innovative Research Group Project of the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
