The effects of remdesivir on mortality and the requirement for mechanical ventilation in patients with COVID-19: a systematic review stratified by disease severity
- PMID: 38151918
- PMCID: PMC10790052
- DOI: 10.3904/kjim.2023.357
The effects of remdesivir on mortality and the requirement for mechanical ventilation in patients with COVID-19: a systematic review stratified by disease severity
Abstract
Background/aims: The effectiveness of remdesivir treatment in reducing mortality and the requirement for mechanical ventilation (MV) remains uncertain, as randomized controlled trials (RCTs) have produced conflicting results.
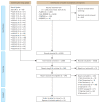
Methods: We searched MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and other data resources to find RCTs published prior to April 10, 2023. The selection of studies, assessment of risk of bias, and meta-analysis were conducted according to PRISMA guidelines. The primary outcomes were all-cause mortality and the need to initiate MV.
Results: A total of 5,068 articles were screened, from eight RCTs comprising 11,945 patients. The meta-analysis found that, compared to standard care or placebo, remdesivir treatment provided no significant all-cause mortality benefit (pooled risk ratio [RR], 0.93; 95% confidence interval [CI], 0.85-1.02; 8 studies; high certainty evidence), while subgroup analyses revealed a trend towards reduced mortality among patients requiring oxygen but not MV (pooled RR, 0.88; 95% CI, 0.77-1.00; 6 studies; I2 = 4%). The need to initiate MV (pooled RR, 0.74; 95% CI, 0.59-0.94; 7 studies; moderate certainty evidence) in remdesivir-treated patients was also reduced compared to controls. Remdesivir significantly increased clinical improvement and discharge and significantly reduced serious adverse events.
Conclusion: In this systematic review and meta-analysis of RCTs, it was found that remdesivir treatment did not show a substantial decrease in the risk of mortality. However, it was linked to a reduction in the necessity for additional ventilatory support, suggesting remdesivir could be beneficial for COVID-19 patients, particularly those who are not on MV.
Keywords: COVID-19; COVID-19 drug treatment; Mortality; Systematic review; Ventilators, mechanical.
Conflict of interest statement
The author discloses no conflicts.
Figures



Similar articles
-
Interventions for treatment of COVID-19: Second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project).PLoS One. 2021 Mar 11;16(3):e0248132. doi: 10.1371/journal.pone.0248132. eCollection 2021. PLoS One. 2021. PMID: 33705495 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 20;5(5):CD013600. doi: 10.1002/14651858.CD013600.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Feb 1;2:CD013600. doi: 10.1002/14651858.CD013600.pub5. PMID: 34013969 Free PMC article. Updated.
-
Interventions for treatment of COVID-19: A living systematic review with meta-analyses and trial sequential analyses (The LIVING Project).PLoS Med. 2020 Sep 17;17(9):e1003293. doi: 10.1371/journal.pmed.1003293. eCollection 2020 Sep. PLoS Med. 2020. PMID: 32941437 Free PMC article.
-
Efficacy and harms of remdesivir for the treatment of COVID-19: A systematic review and meta-analysis.PLoS One. 2020 Dec 10;15(12):e0243705. doi: 10.1371/journal.pone.0243705. eCollection 2020. PLoS One. 2020. PMID: 33301514 Free PMC article.
-
Remdesivir for the treatment of COVID-19: a living systematic review.Medwave. 2020 Dec 9;20(11):e8080. doi: 10.5867/medwave.2020.11.8080. Medwave. 2020. PMID: 33361753 English.
Cited by
-
SARS-CoV-2 viral load is linked to remdesivir efficacy in severe Covid-19 admitted to intensive care.Sci Rep. 2024 Sep 6;14(1):20825. doi: 10.1038/s41598-024-71588-9. Sci Rep. 2024. PMID: 39242658 Free PMC article.
References
-
- Ali K, Azher T, Baqi M, et al. Canadian Treatments for COVID-19 (CATCO); Association of Medical Microbiology and Infectious Disease Canada (AMMI) Clinical Research Network and the Canadian Critical Care Trials Group Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. CMAJ. 2022;194:E242–E251. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
