TLR3 agonism augments CD47 inhibition in acute myeloid leukemia
- PMID: 38152031
- PMCID: PMC11215363
- DOI: 10.3324/haematol.2023.283850
TLR3 agonism augments CD47 inhibition in acute myeloid leukemia
Abstract
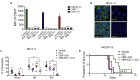
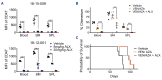
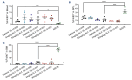
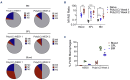
CD47-SIRPa is a myeloid check point pathway that promotes phagocytosis of cells lacking markers for self-recognition. Tumor cells can overexpress CD47 and bind to SIRPa on macrophages, preventing phagocytosis. CD47 expression is enhanced and correlated with a negative prognosis in acute myeloid leukemia (AML), with its blockade leading to cell clearance. ALX90 is an engineered fusion protein with high affinity for CD47. Composed of the N-terminal D1 domain of SIRPα genetically linked to an inactive Fc domain from human immunoglobulin (Ig) G, ALX90 is designed to avoid potential toxicity of CD47-expressing red blood cells. Venetoclax (VEN) is a specific B-cell lymphoma-2 (BCL-2) inhibitor that can restore apoptosis in malignant cells. In AML, VEN is combined with azanucleosides to induce superior remission rates, however treatment for refractory/relapse is an unmet need. We questioned whether the anti-tumor activity of a VENbased regimen can be augmented through CD47 inhibition (CD47i) in AML and how this triplet may be enhanced. Human AML cell lines were sensitive to ALX90 and its addition increased efficacy of a VEN plus azacitidin (VEN+AZA) regimen in vivo. However, CD47i failed to clear bone marrow tumor burden in PDX models. We hypothesized that the loss of resident macrophages in the bone marrow in AML reduced efficiency of CD47i. Therefore, we attempted to enhance this medullary macrophage population with agonism of TLR3 via polyinosinic:polycytidylic acid (poly(I:C)), which led to expansion and activation of medullary macrophages in in vivo AML PDX models and potentiated CD47i. In summary, the addition of poly(I:C) can enhance medullary macrophage populations to potentiate the phagocytosis merited by therapeutic inhibition of CD47.
Figures




Similar articles
-
Novel fully human anti-CD47 antibodies stimulate phagocytosis and promote elimination of AML cells.J Cell Physiol. 2021 Jun;236(6):4470-4481. doi: 10.1002/jcp.30163. Epub 2020 Nov 18. J Cell Physiol. 2021. PMID: 33206395
-
Modulation of CD47-SIRPα innate immune checkpoint axis with Fc-function detuned anti-CD47 therapeutic antibody.Cancer Immunol Immunother. 2022 Feb;71(2):473-489. doi: 10.1007/s00262-021-03010-6. Epub 2021 Jul 10. Cancer Immunol Immunother. 2022. PMID: 34247273 Free PMC article.
-
Blockade of the CD47/SIRPα checkpoint axis potentiates the macrophage-mediated antitumor efficacy of tafasitamab.Haematologica. 2024 Dec 1;109(12):3928-3940. doi: 10.3324/haematol.2023.284795. Haematologica. 2024. PMID: 38934068 Free PMC article.
-
Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis.Front Immunol. 2020 Jan 28;11:18. doi: 10.3389/fimmu.2020.00018. eCollection 2020. Front Immunol. 2020. PMID: 32082311 Free PMC article. Review.
-
Cancer immunotherapy targeting the CD47/SIRPα axis.Eur J Cancer. 2017 May;76:100-109. doi: 10.1016/j.ejca.2017.02.013. Epub 2017 Mar 10. Eur J Cancer. 2017. PMID: 28286286 Review.
Cited by
-
Pathogenesis and inflammaging in myelodysplastic syndrome.Haematologica. 2025 Feb 1;110(2):283-299. doi: 10.3324/haematol.2023.284944. Haematologica. 2025. PMID: 39445405 Free PMC article. Review.
-
Toll-Like Receptors in the Immunotherapy Era: Dual-Edged Swords of Tumor Immunity and Clinical Translation.MedComm (2020). 2025 Jul 27;6(8):e70308. doi: 10.1002/mco2.70308. eCollection 2025 Aug. MedComm (2020). 2025. PMID: 40727252 Free PMC article. Review.
-
Toll-like receptor 3: a double-edged sword.Biomark Res. 2025 Feb 23;13(1):32. doi: 10.1186/s40364-025-00739-5. Biomark Res. 2025. PMID: 39988665 Free PMC article. Review.
-
Investigating resistance to 5-Azacytidine and Venetoclax in PDX models of MDS/AML.Front Oncol. 2025 Jan 7;14:1414950. doi: 10.3389/fonc.2024.1414950. eCollection 2024. Front Oncol. 2025. PMID: 39839764 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

