Prefrontal cortex glutamatergic adaptations in a mouse model of alcohol use disorder
- PMID: 38152067
- PMCID: PMC10752437
- DOI: 10.1016/j.addicn.2023.100137
Prefrontal cortex glutamatergic adaptations in a mouse model of alcohol use disorder
Abstract
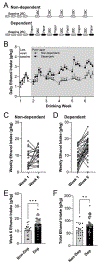
Alcohol use disorder (AUD) produces cognitive deficits, indicating a shift in prefrontal cortex (PFC) function. PFC glutamate neurotransmission is mostly mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type ionotropic receptors (AMPARs); however preclinical studies have mostly focused on other receptor subtypes. Here we examined the impact of early withdrawal from chronic ethanol on AMPAR function in the mouse medial PFC (mPFC). Dependent male C57BL/6J mice were generated using the chronic intermittent ethanol vapor-two bottle choice (CIE-2BC) paradigm. Non-dependent mice had access to water and ethanol bottles but did not receive ethanol vapor. Naïve mice had no ethanol exposure. We used patch-clamp electrophysiology to measure glutamate neurotransmission in layer 2/3 prelimbic mPFC pyramidal neurons. Since AMPAR function can be impacted by subunit composition or plasticity-related proteins, we probed their mPFC expression levels. Dependent mice had higher spontaneous excitatory postsynaptic current (sEPSC) amplitude and kinetics compared to the Naïve/Non-dependent mice. These effects were seen during intoxication and after 3-8 days withdrawal, and were action potential-independent, suggesting direct enhancement of AMPAR function. Surprisingly, 3 days withdrawal decreased expression of genes encoding AMPAR subunits (Gria1/2) and synaptic plasticity proteins (Dlg4 and Grip1) in Dependent mice. Further analysis within the Dependent group revealed a negative correlation between Gria1 mRNA levels and ethanol intake. Collectively, these data establish a role for mPFC AMPAR adaptations in the glutamatergic dysfunction associated with ethanol dependence. Future studies on the underlying AMPAR plasticity mechanisms that promote alcohol reinforcement, seeking, drinking and relapse behavior may help identify new targets for AUD treatment.
Keywords: AMPA; Addiction; Excitatory transmission; GluR1; Neuroplasticity.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



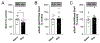

Similar articles
-
Increased Synaptic Strength and mGlu2/3 Receptor Plasticity on Mouse Prefrontal Cortex Intratelencephalic Pyramidal Cells Following Intermittent Access to Ethanol.Alcohol Clin Exp Res. 2021 Mar;45(3):518-529. doi: 10.1111/acer.14546. Epub 2021 Feb 9. Alcohol Clin Exp Res. 2021. PMID: 33434325 Free PMC article.
-
Effects of acamprosate on attentional set-shifting and cellular function in the prefrontal cortex of chronic alcohol-exposed mice.Alcohol Clin Exp Res. 2015 Jun;39(6):953-61. doi: 10.1111/acer.12722. Epub 2015 Apr 23. Alcohol Clin Exp Res. 2015. PMID: 25903298 Free PMC article.
-
Do Alcohol-Related AMPA-Type Glutamate Receptor Adaptations Promote Intake?Handb Exp Pharmacol. 2018;248:157-186. doi: 10.1007/164_2018_105. Handb Exp Pharmacol. 2018. PMID: 29675583
-
Role of nucleus accumbens glutamatergic plasticity in drug addiction.Neuropsychiatr Dis Treat. 2013;9:1499-512. doi: 10.2147/NDT.S45963. Epub 2013 Sep 30. Neuropsychiatr Dis Treat. 2013. PMID: 24109187 Free PMC article. Review.
-
Targeting prefrontal cortex GABAergic microcircuits for the treatment of alcohol use disorder.Front Synaptic Neurosci. 2022 Aug 29;14:936911. doi: 10.3389/fnsyn.2022.936911. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 36105666 Free PMC article. Review.
Cited by
-
Pre- and postsynaptic signatures in the prelimbic cortex associated with "alcohol use disorder" in the rat.Neuropsychopharmacology. 2024 Nov;49(12):1851-1860. doi: 10.1038/s41386-024-01887-2. Epub 2024 May 16. Neuropsychopharmacology. 2024. PMID: 38755284 Free PMC article.
-
Altered Expression of Neuroplasticity-Related Genes in Alcohol Addiction and Treatment.Int J Mol Sci. 2024 Oct 22;25(21):11349. doi: 10.3390/ijms252111349. Int J Mol Sci. 2024. PMID: 39518903 Free PMC article.
-
Ethanol's impact on the brain: a neurobiological perspective on the mechanisms of memory impairment.Mol Biol Rep. 2024 Jun 25;51(1):782. doi: 10.1007/s11033-024-09748-3. Mol Biol Rep. 2024. PMID: 38918289 Review.
-
Functional and morphological adaptation of medial prefrontal corticotropin releasing factor receptor 1-expressing neurons in male mice following chronic ethanol exposure.Neurobiol Stress. 2024 Jun 17;31:100657. doi: 10.1016/j.ynstr.2024.100657. eCollection 2024 Jul. Neurobiol Stress. 2024. PMID: 38983690 Free PMC article.
References
-
- Hu Y, et al., Glutamate levels in the ventromedial prefrontal cortex and resting-state functional connectivity within reward circuits in alcohol-dependent patients, Addict. Biol 28 (2023) e13272. - PubMed
Grants and funding
- U01 AA013498/AA/NIAAA NIH HHS/United States
- R01 AA025996/AA/NIAAA NIH HHS/United States
- R33 AA027636/AA/NIAAA NIH HHS/United States
- R37 AA017447/AA/NIAAA NIH HHS/United States
- K99 AA025408/AA/NIAAA NIH HHS/United States
- R01 AA017447/AA/NIAAA NIH HHS/United States
- P50 AA017823/AA/NIAAA NIH HHS/United States
- P50 AA006420/AA/NIAAA NIH HHS/United States
- R01 AA027700/AA/NIAAA NIH HHS/United States
- R01 AA026685/AA/NIAAA NIH HHS/United States
- R21 AA031101/AA/NIAAA NIH HHS/United States
- R00 AA025408/AA/NIAAA NIH HHS/United States
- R01 AA029841/AA/NIAAA NIH HHS/United States
- R21 AA027636/AA/NIAAA NIH HHS/United States
- R01 AA021491/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
