Feasibility and value of a domiciliary spirometry programme in the assessment of severe asthma: a real-world evaluation
- PMID: 38152082
- PMCID: PMC10752269
- DOI: 10.1183/23120541.00635-2023
Feasibility and value of a domiciliary spirometry programme in the assessment of severe asthma: a real-world evaluation
Abstract
Background: Domiciliary spirometry (DS) is a novel tool that is widely employed in the assessment of respiratory disease. We assessed real-world feasibility, effectiveness and value of a physiologist-led home spirometry programme in patients with treatment-refractory severe asthma.
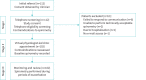
Methods: Patients were referred and provided with a hand-held DS device. Patients completed baseline measurements in a physiologist-led virtual clinic and were instructed to provide further values during any periods of respiratory symptoms. Outcome measures included prevalence of new obstructed events, DS adherence and uptake of this approach.
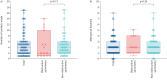
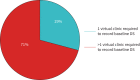
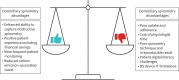
Results: 112 patients were enrolled from November 2020 to January 2023. 102 individuals, mean±sd age 44±13 years (86% female) with median (IQR) forced expiratory volume in 1 s % predicted 88% (77-97%), successfully recorded baseline spirometry values. During follow-up (24 months), 11 (11%) were identified with new obstructive spirometry and were subsequently able to be commenced on biologic therapy. Patient engagement was poor with median (IQR) of 4 (2-6) attempts of contact made before baseline values were recorded, and 2 (1-3) attempts required to record technically acceptable values. Continued DS use was suboptimal; 34% failed to use their device after baseline and only 10% continued at the end of the study period. The cost of DS measurements was greater than a single hospital-based visit but enables multiple event capture.
Conclusion: Overall, DS measurement uptake was poor, with a minority of patients continuing to use the device at the end of the study period. However, for those that engage, DS provides an alternative approach to traditional hospital-based spirometry measurements that can alter clinical management.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: Z. Williams, Y. Ge, J. Ming, C. Roberts and S. Rhamie have no conflict of interests to declare. Conflict of interest: J.H. Hull is an associate editor of this journal. Conflict of interest: P.H. Patel reports attending advisory boards for AstraZeneca, Celltrion Healthcare and GlaxoSmithKline; he has received speaker fees from AstraZeneca, GlaxoSmithKline, Novartis, and Sanofi/Regeneron; he has attended international conferences sponsored by AstraZeneca.
Figures
References
-
- Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. 2022.. Date last accessed: 16 August 2023. https://ginasthma.org/
LinkOut - more resources
Full Text Sources
