Androgen excess: a hallmark of polycystic ovary syndrome
- PMID: 38152131
- PMCID: PMC10751361
- DOI: 10.3389/fendo.2023.1273542
Androgen excess: a hallmark of polycystic ovary syndrome
Abstract
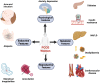
Polycystic ovarian syndrome (PCOS) is a metabolic, reproductive, and psychological disorder affecting 6-20% of reproductive women worldwide. However, there is still no cure for PCOS, and current treatments primarily alleviate its symptoms due to a poor understanding of its etiology. Compelling evidence suggests that hyperandrogenism is not just a primary feature of PCOS. Instead, it may be a causative factor for this condition. Thus, figuring out the mechanisms of androgen synthesis, conversion, and metabolism is relatively important. Traditionally, studies of androgen excess have largely focused on classical androgen, but in recent years, adrenal-derived 11-oxygenated androgen has also garnered interest. Herein, this Review aims to investigate the origins of androgen excess, androgen synthesis, how androgen receptor (AR) signaling mediates adverse PCOS traits, and the role of 11-oxygenated androgen in the pathophysiology of PCOS. In addition, it provides therapeutic strategies targeting hyperandrogenism in PCOS.
Keywords: Hyperandrogenism; androgen receptor; insulin resistance; polycystic ovarian syndrome; steroidogenesis.
Copyright © 2023 Wang, Li and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

